| Identification | More | [Name]
L-Serine benzyl ester hydrochloride | [CAS]
60022-62-0 | [Synonyms]
H-SER-OBZL HCL
L-SERINE BENZYL ESTER HYDROCHLORIDE
SERINE-OBZL HCL
L-SerinebenzylesterHCl | [Molecular Formula]
C10H14ClNO3 | [MDL Number]
MFCD00038955 | [Molecular Weight]
231.68 | [MOL File]
60022-62-0.mol |
| Chemical Properties | Back Directory | [Melting point ]
175°C | [refractive index ]
-12 ° (C=1, H2O) | [storage temp. ]
−20°C
| [form ]
powder to crystal | [color ]
White to Almost white | [Water Solubility ]
Soluble in water. | [InChI]
InChI=1/C10H13NO3.ClH/c11-9(6-12)10(13)14-7-8-4-2-1-3-5-8;/h1-5,9,12H,6-7,11H2;1H/t9-;/s3 | [InChIKey]
MGZWCDQAKCHOBX-FVGYRXGTSA-N | [SMILES]
C(OCC1=CC=CC=C1)(=O)[C@H](CO)N.[H]Cl |&1:10,r| | [CAS DataBase Reference]
60022-62-0(CAS DataBase Reference) |
| Hazard Information | Back Directory | [Description]
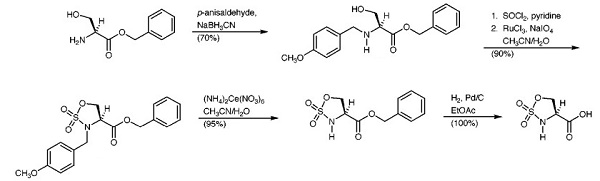
L-Serine benzyl ester hydrochloride, also known as L-Serine-Bz-HCl, is a derivative of the amino acid L-Serine. It is a water-soluble, white crystalline powder and is commonly used as a reagent in biochemical and physiological studies. It is a useful raw material that could synthesize mang organic compounds, such as a cyclic sulfamidate which could reacted cleanly with the sodium thiolate
salt of a variety of unprotected 1-thio sugars in aqueous buffer to afford the corresponding S-linked amino acid glycoconjugates in good
yields after hydrolysis of the N-sulfates[1].
| [Chemical Properties]
Colorless solid | [Uses]
It is an important raw material and intermediate used in organic synthesis, pharmaceuticals, agrochemicals and dyestuff. | [Preparation]
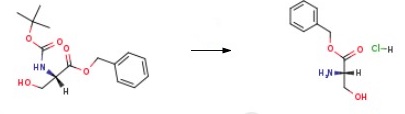
Boc-L-Ser-OBz (2.953 g, 10 mmol) was dissolved in MeOH (21 mL) and was palced in ice bath. Then concentrated hydrochloric acid (4.25 mL) was added dropwise. The ice bath was removed and the reaction mixture was stirred for 3 hours. After that the isopropanol (10 mL) was added and the volatile components were removed under reduced pressure at 55 ℃. The oily residue was coevaporated with isopropanol three times (3x10 mL). L-Serine benzyl ester hydrochloride ( yield=69%) was crystallized from H2O/iPr-OH/acetone mixture.
 | [References]
[1] Cohen S, et al. Synthesis of S-linked glycosyl amino acids in aqueous solution with unprotected carbohydrates. Organic Letters, 2001; 3: 405–407. |
|
|