| Identification | More | [Name]
Vidarabine | [CAS]
5536-17-4 | [Synonyms]
2'-ARAADENOSINE
6-AMINO-9-BETA-D-ARABINOFURANOSYLPURINE
9-BETA-D-ARABINOFURANOSYLADENINE
9-BETA-D-ARABINOSYLADENINE
ADENINE-9-BETA-D-ARABINOFURANOSIDE
ADENINE-BETA-D-ARABINOFURANOSIDE
ARA-A
ARABINOSYL-ADENINE
SPONGOADENOSINE
VIDARABINE
9-arabinosyladenine
9-beta-d-arabinofuranosyl-9h-purin-6-amin
9-beta-d-arabinofuranosyl-adenin
adeninearabinoside
araadenosine
arasena-a
beta-ara-a
beta-d-arabinosyladenine
ci673
ci-673 | [EINECS(EC#)]
226-893-9 | [Molecular Formula]
C10H13N5O4 | [MDL Number]
MFCD00065471 | [Molecular Weight]
267.24 | [MOL File]
5536-17-4.mol |
| Chemical Properties | Back Directory | [Appearance]
Crystalline | [Melting point ]
260-265 °C (dec.) | [alpha ]
D27 -5° (c = 0.25) | [Boiling point ]
410.43°C (rough estimate) | [density ]
1.3382 (rough estimate) | [refractive index ]
1.7610 (estimate) | [storage temp. ]
−20°C
| [solubility ]
DMSO (Slightly, Heated) | [form ]
Powder | [pka]
pKa 3.55(H2O
t=20
I=0.1 (KCl)) (Uncertain);11.4 (Uncertain) | [color ]
White to Off-white | [Water Solubility ]
Soluble in DMF (10 mg/ml), 0.5 M HCl (50 mg/ml), DMSO (53 mg/ml at 25°C), ethanol (<1 mg/ml at 25°C), and water (3 mg/ml at 25°C). | [Merck ]
13,10039 | [BRN ]
624881 | [InChIKey]
OIRDTQYFTABQOQ-UHTZMRCNSA-N | [LogP]
-0.755 (est) | [CAS DataBase Reference]
5536-17-4(CAS DataBase Reference) | [EPA Substance Registry System]
5536-17-4(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
Xn,Xi | [Risk Statements ]
R63:Possible risk of harm to the unborn child.
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S36/37:Wear suitable protective clothing and gloves .
S36:Wear suitable protective clothing .
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice . | [RIDADR ]
2811 | [WGK Germany ]
3
| [RTECS ]
AU6200000
| [F ]
10-23 | [TSCA ]
Yes | [HazardClass ]
6.1(a) | [PackingGroup ]
II | [HS Code ]
29349990 | [Safety Profile]
Poison by ingestion and
intravenous routes. Moderately toxic by
intraperitoneal route. An experimental
teratogen. Other experimental reproductive
effects. Human systemic effects by
intravenous route: central nervous system,
blood, and other effects. A skin and eye
irritant. Human mutation data reported.
When heated to decomposition it emits
toxic fumes of NOx. | [Hazardous Substances Data]
5536-17-4(Hazardous Substances Data) | [Toxicity]
LD50 in mice (mg/kg): 4677 i.p.; >7950 orally (Kurtz) |
| Hazard Information | Back Directory | [General Description]
White to off-white crystalline powder. | [Reactivity Profile]
VIDARABINE(5536-17-4) is an aminoalcohol. Amines are chemical bases. They neutralize acids to form salts plus water. These acid-base reactions are exothermic. The amount of heat that is evolved per mole of amine in a neutralization is largely independent of the strength of the amine as a base. Amines may be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen is generated by amines in combination with strong reducing agents, such as hydrides. | [Air & Water Reactions]
Insoluble in water. | [Fire Hazard]
Flash point data for this chemical are not available; however VIDARABINE is probably combustible. | [Description]
Vidarabine (adenine arabinoside) is the stereoisomer of adenosine. This analog of a purine
nucleoside exhibits selective activity against the herpes virus. The ribose residue is replaced
with an arabinose residue. Like acyclovir, it turns into mono-, di-, and triphosphate in cells
infected by a virus, thus inhibiting DNA polymerase, and correspondingly preventing DNA
synthesis of the virus approximately 20–40 times more than in “host” cells. It is easily metab�olized to a less active, yet nonetheless antiviral compound—arabinosylhypoxanthine. It has
been successfully used for herpetic encephalitis, and for complicated shingles. It is used in
the form of eye drops for herpetic keratoconjuctivitis. A synonym of this drug is Vira-A. | [Description]
Vidarabine is an analog of the nucleoside adenosine that has antiviral properties. It acts as a prodrug that, once phosphorylated by cellular enzymes, acts as both substrate and inhibitor of DNA polymerase.1 Vidarabine is particularly effective against H. simplex and V. zoster viruses.1 | [Chemical Properties]
Crystalline | [Originator]
Vidarabin ,Thilo,W. Germany ,1975 | [Uses]
active component of chili peppers, analgesic and therapeutic agent for arthritis, potential prophylactic for type 1 diabetes | [Uses]
antifungal;Antiviral;Adenosine antimetabolite. | [Uses]
Vidarabine, is an antiviral drug which is active against herpes simplex and varicella zoster viruses. | [Definition]
ChEBI: Adenine arabinoside is a purine nucleoside in which adenine is attached to arabinofuranose via a beta-N(9)-glycosidic bond. It has a role as an antineoplastic agent, a bacterial metabolite and a nucleoside antibiotic. It is a purine nucleoside and a beta-D-arabinoside. It is functionally related to an adenine. | [Indications]
Vidarabine (adenine arabinoside, Vira-A) is an adenine
nucleoside analogue containing arabinose in place of ribose.
It is obtained from cultures of Streptomyces antibioticus
and has activity against HSV-1, HSV-2, VZV,
CMV, HBV, poxviruses, hepadnaviruses, rhabdoviruses,
and certain RNA tumor viruses. | [Manufacturing Process]
Sterile agar slants are prepared using the Streptomyces sporulation medium
of Hickey and Tresner, J. Bact., vol. 64, pages 891-892 (1952). Four of these
slants are inoculated with lyophilized spores of Streptomyces antibioticus
NRRL 3238, incubated at 28°C for 7 days or until aerial spore growth is well-
advanced, and then stored at 5°C. The spores from the four slants are
suspended in 40 ml of 0.1% sterile sodium heptadecyl sulfate solution. A
nutrient medium having the following composition is then prepared: 2.0%
glucose monohydrate; 1.0% soybean meal, solvent extracted, 44% protein;
0.5% animal peptone (Wilson's protopeptone 159); 0.2% ammonium chloride;
0.5% sodium chloride; 0.25% calcium carbonate; and water to make 100%.
The pH of the medium is adjusted with 10-normal sodium hydroxide solution
to pH 7.5. 12 liters of this medium is placed in a 30-liter stainless steel
fermenter. The medium is sterilized by heating it at 121°C for 90 minutes,
allowed to cool, inoculated with the 40 ml spore suspension described above,
and incubated at 25° to 27°C for 32 hours while being agitated at 200 rpm
with air being supplied at the rate of 12 liters per minute. About 38 grams of
a mixture of lard and mineral oils containing mono-and diglycerides is added
in portions during this time to prevent excessive foaming.
16 liters of a nutrient medium having the composition described above is
placed in each of four 30-liter stainless steel fermenters. The pH of the medium in each fermenter is adjusted with 10-normal sodium hydroxide
solution to pH 7.5, and each is sterilized by heating at 121°C for 90 minutes.
Upon cooling, the medium in each fermenter is inoculated with 800 ml of the
fermentation mixture described above, and each is incubated at 25° to 27°C
for 96 hours while being agitated at 200 rpm with air being supplied at the
rate of 16 liters per minute. About 170 grams of the antifoam mixture
described above is added in portions during this time to the medium in each
fermenter.
The fermentation mixtures from the four fermenters are combined and filtered
with the aid of diatomaceous earth, A material such as Celite 545 can be
used. The filtrate is concentrated under reduced pressure to a volume of 10
liters, and the concentrate is treated with 200 grams of activated charcoal (for
example, Darco G-60), stirred at room temperature for one hour, and filtered.
The charcoal cake is washed with 7.5 liters of water, and then extracted with
three 10-liter portions of 50% aqueous acetone. The three aqueous acetone
extracts are combined, concentrated under reduced pressure to approximately
one liter, and chilled at 5°C for 48 hours. The solid 9-(β-D-
arabinofuranosyl)adenine that precipitates is isolated and purified by
successive crystallizations from boiling methanol and from boiling water; MP
262° to 263°C.
In the foregoing procedure, when the temperature of incubation in the two
fermentation stages is raised from 25° to 27°C to 36° to 38°C, the same 9-
(β-D-arabinofuranosyl)adenine product is obtained in higher yields. | [Brand name]
Vira-A (Parkdale). | [Therapeutic Function]
Antiviral | [Biochem/physiol Actions]
Cell-permeable adenylate cyclase inhibitor; in detergent-dispersed rat brain preparation, IC50 = 30 μM. Clinically significant antiviral agent, especially against herpes simplex (HSV), by inhibition of DNA polymerase. | [Mechanism of action]
Vidarabine’s specific mechanism of action is not
fully understood. Cellular enzymes convert this drug to
a triphosphate that inhibits DNA polymerase activity.
Vidarabine triphosphate competes with deoxyadenosine
triphosphate (dATP) for access to DNA polymerase
and also acts as a chain terminator. Although
vidarabine is incorporated into host DNA to some extent,
viral DNA polymerase is much more susceptible to
inhibition by vidarabine. Vidarabine also inhibits ribonucleoside
reductase and other enzymes. Resistance
occurs as a result of DNA polymerase mutation. | [Pharmacokinetics]
Vidarabine is deaminated rapidly by adenosine deaminase, which is present in serum and red blood cells.
The enzyme converts vidarabine to its principal metabolite, arabinosyl hypoxanthine (ara-HX), which has
weak antiviral activity. The half-life of vidarabine is approximately 1 hour, whereas
ara-HX has a half-life of 3.5 hours. The drug is detected mostly in the kidney, liver, and spleen, because
50% of it is recovered in the urine as ara-HX. Levels of vidarabine in CSF fluid are 50% of those in the
plasma. | [Clinical Use]
The principal use of vidarabine is in the treatment of
HSV keratoconjunctivitis. It is also used to treat superficial
keratitis in patients unresponsive or hypersensitive
to topical idoxuridine. | [Side effects]
The most commonly observed side effects associated
with vidarabine are lacrimation, burning, irritation,
pain, and photophobia. Vidarabine has oncogenic and
mutagenic potential; however, the risk of systemic effects
is low because of its limited absorption. It should
not be used in conjunction with ophthalmic corticosteroids,
since these drugs increase the spread of HSV
infection and may produce side effects such as increased
intraocular pressure, glaucoma, and cataracts. | [Synthesis]
Vidarabine, 9-B-arabinofuranosyl-6-amino-9-H-pyrine (36.1.10), is synthesized both microbiologically from the culture fluid of the actinomycete Streptomyces antibioticus NRRL 3238, as well as synthetically. It is synthesized from the acetonide-β-D–xylofuranoside of adenine—9-(3�,5�-O-isopropyliden-β-D–xylofuranoside)adenine, which is reacted with methanesulfonyl chloride to make the mesylate 9-(3,5-O-isopropyliden-2-O-methansulfonyl-β-D-xydlofuranoside)adenine (36.1.7). Prolonged heating in 90% acetic acid removes the acetonyl protective group from the resulting compound, giving the product (36.1.8).
Reacting this with sodium methoxide leads to the formation of an epoxide— 9-(2,3-anhydro-β-luxofuranosyl)adenine (36.1.9). Finally, heating this epoxide with sodium acetate or benzoate opens the epoxide ring in the dimethylformamide–water system to make the corresponding dihydroxy derivative, vidarabine.
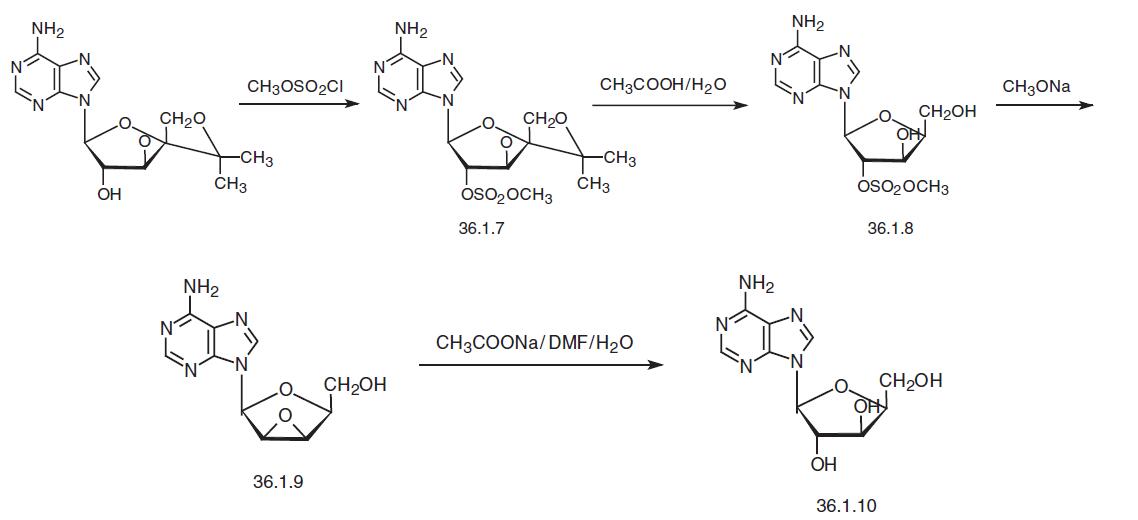
Another way of synthesis of vidarabine that was developed later consists of alkylating of 6-benzamidopurine with 2,3,5-tri-O-benzyl-D-arabinofuranosyl chloride using sodium in liquid ammonia. This simultaneously N-debenzylates the sixth position of the purine sys�tem and fulfil O-debenzylation of hydroxyl groups of the furanosyl fragment of the molu�cule, giving vidarabine.

|
|
|