| Identification | More | [Name]
TRI(2-FURYL)PHOSPHINE | [CAS]
5518-52-5 | [Synonyms]
SALOR-INT L308749-1EA
TRI(2-FURYL)PHOSPHINE
TRIS(2-FURYL)PHOSPHINE
Tri(2-furyl)phosphine,97%
Tri-2-furylphosphine,98+%
TRI-(2-FURYL)PHOSPHINE 98%
2,2',2''-Phosphinidynetrisfuran
Tri(2-furanyl)phosphine
Tris(2-furanyl)phosphine
Tris(furan-2-yl)phosphine | [EINECS(EC#)]
628-036-8 | [Molecular Formula]
C12H9O3P | [MDL Number]
MFCD00151857 | [Molecular Weight]
232.17 | [MOL File]
5518-52-5.mol |
| Chemical Properties | Back Directory | [Appearance]
white to light yellow crystal powde | [Melting point ]
59-64 °C (lit.) | [Boiling point ]
136 °C/4 mmHg (lit.) | [Fp ]
136°C/4mm | [storage temp. ]
Keep in dark place,Sealed in dry,Room Temperature | [form ]
solid | [color ]
faintly brown
| [Sensitive ]
air sensitive | [BRN ]
1577564 | [InChI]
InChI=1S/C12H9O3P/c1-4-10(13-7-1)16(11-5-2-8-14-11)12-6-3-9-15-12/h1-9H | [InChIKey]
DLQYXUGCCKQSRJ-UHFFFAOYSA-N | [SMILES]
P(C1=CC=CO1)(C1=CC=CO1)C1=CC=CO1 | [CAS DataBase Reference]
5518-52-5(CAS DataBase Reference) |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S24/25:Avoid contact with skin and eyes . | [WGK Germany ]
3
| [F ]
10-23 | [TSCA ]
No | [HS Code ]
29319090 |
| Hazard Information | Back Directory | [Chemical Properties]
TRI(2-FURYL)PHOSPHINE is white to light yellow crystal powde
| [Uses]
suzuki reaction | [Uses]
TRI(2-FURYL)PHOSPHINE is a phopshine ligand used in transition-metal mediated organic synthesis, in particular in wittig reactions to improved (Z) selectivity.
| [reaction suitability]
reagent type: ligand
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reagent type: ligand
reaction type: Cycloisomerizations
reagent type: ligand
reaction type: Heck Reaction
reagent type: ligand
reaction type: Negishi Coupling
reagent type: ligand
reaction type: Oxidations
reagent type: ligand
reaction type: Sonogashira Coupling
reagent type: ligand
reaction type: Stille Coupling
reagent type: ligand
reaction type: Suzuki-Miyaura Coupling |
| Questions And Answer | Back Directory | [Reaction]
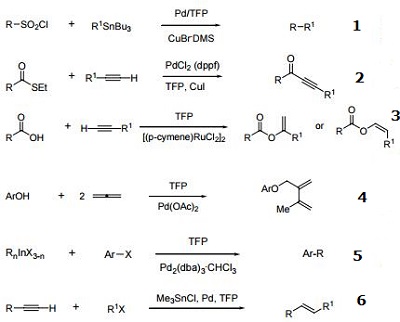
- Useful ligand for C-C coupling reactions.
- Ligand used for the alkynylation of thioesters.
- Ligand used for enol ester formation.
- Ligand for palladium-catalyzed 3-Component coupling.
- Ligand for palladium-catalyzed C-C coupling reaction.
- Ligand for trans-olefin formation.
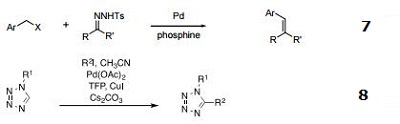
- Olefin formation from N-tosylhydrazones and benzyl halides.
- C-H arylation/alkenylation of 1-substituted tetrazoles.
|
|
|