| Identification | More | [Name]
Crystal Violet | [CAS]
548-62-9 | [Synonyms]
BASIC VIOLET 11:1
BASIC VIOLET 3
BRILLIANT VIOLET
CALCOZINE VIOLET 6BN
CI 42535
CI 42555
CI NO 42555
CI NO 42555/42535
CRYSTAL (GENTIAN) VIOLET
CRYSTAL VIOLET
CRYSTAL VIOLET BASE
GENTIAN VIOLET
GENTIAN VIOLET 10B
GENTIAN VIOLET 2
GENTIAN VIOLET 3
GENTIAN VIOLET B
GENTIAN VIOLET, GRAMS
GENTIAN VIOLET, HUCKER
GENTIAN VIOLET, HUCKER'S
GRAM STAIN | [EINECS(EC#)]
208-953-6 | [Molecular Formula]
C25H34Cl2N4 | [MDL Number]
MFCD00011750 | [Molecular Weight]
461.47 | [MOL File]
548-62-9.mol |
| Chemical Properties | Back Directory | [Appearance]
dark green powder or crystals | [Melting point ]
205 °C (dec.) (lit.) | [Boiling point ]
560.86°C (rough estimate) | [bulk density]
220-400kg/m3 | [density ]
1.19 g/cm3 at 20 °C | [vapor pressure ]
0Pa at 25℃ | [refractive index ]
1.6010 (estimate) | [Fp ]
40 °C | [storage temp. ]
Store at RT. | [solubility ]
water: soluble50g/L at 27°C | [Colour Index ]
42555 | [form ]
Solid | [pka]
9.4(at 25℃) | [color ]
S. No.: 785 | [Odor]
Slight characteristic odor | [PH]
2.5-3.5 (10g/l, H2O, 20℃) | [PH Range]
0.8(yellow)-2.6(blue/violet) | [Stability:]
Stable. Incompatible with strong oxidizing agents, strong acids. Light-sensitive. Combustible. | [Water Solubility ]
16 g/L (25 ºC) | [ε(extinction coefficient)]
≥1750 at 585-595 nm in water | [λmax]
590nm | [Merck ]
14,4395 | [BRN ]
4077708 | [Biological Applications]
Detecting microorganisms; treating atopic dermatitis,dermatological diseases,28,skin wounds,lesions,hemorrhoids,1,multiple myeloma,Non-Hodgkin’s lymphoma,breast cancer,neurodegenerative diseases,onychomycosis; wound dressing | [Major Application]
Photoresists, lithographic printing plate, printed circuit board, inks, hair dyes, shampoo, drug screening method, bone cement preparation method, microorganisms, hemorrhoids, antifungal, antibacterial, antimalarial agent, dental application | [InChIKey]
ZXJXZNDDNMQXFV-UHFFFAOYSA-M | [LogP]
1.172 at 25℃ | [CAS DataBase Reference]
548-62-9(CAS DataBase Reference) | [EPA Substance Registry System]
548-62-9(EPA Substance) | [Absorption]
passes test |
| Safety Data | Back Directory | [Hazard Codes ]
Xn,N,T,C | [Risk Statements ]
R52/53:Harmful to aquatic organisms, may cause long-term adverse effects in the aquatic environment .
R50/53:Very Toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment .
R45:May cause cancer.
R41:Risk of serious damage to eyes.
R22:Harmful if swallowed.
R40:Limited evidence of a carcinogenic effect.
R34:Causes burns.
R10:Flammable.
R36/37/38:Irritating to eyes, respiratory system and skin .
R23/25:Toxic by inhalation and if swallowed . | [Safety Statements ]
S61:Avoid release to the environment. Refer to special instructions safety data sheet .
S60:This material and/or its container must be disposed of as hazardous waste .
S53:Avoid exposure-obtain special instruction before use .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) .
S46:If swallowed, seek medical advice immediately and show this container or label .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S39:Wear eye/face protection .
S36/37:Wear suitable protective clothing and gloves .
S16:Keep away from sources of ignition-No smoking .
S22:Do not breathe dust . | [RIDADR ]
UN 3077 9/PG 3
| [WGK Germany ]
3
| [RTECS ]
BO9000000
| [TSCA ]
Yes | [HazardClass ]
9 | [PackingGroup ]
III | [HS Code ]
32041300 | [Safety Profile]
Poison by ingestion,
intravenous, and intraperitoneal routes, An
experimental teratogen. Other experimental
reproductive effects. A human sktn irritant.
Human mutation data reported.
Questionable carcinogen with experimental
carcinogenic data. When heated to
decomposition it emits very toxic fumes of
NO, and Cl-. | [Hazardous Substances Data]
548-62-9(Hazardous Substances Data) | [Toxicity]
LD50 orally in mice, rats: 1.2, 1.0 g/kg (Hodge) |
| Hazard Information | Back Directory | [General Description]
Green to dark green powder. | [Reactivity Profile]
HEXAMETHYL-P-ROSANILINE CHLORIDE(548-62-9) is light sensitive. . May react vigorously with strong oxidizing agents. May react exothermically with reducing agents to release gaseous hydrogen. | [Air & Water Reactions]
Insoluble in water. | [Fire Hazard]
Flash point data for this compound are not available, however, HEXAMETHYL-P-ROSANILINE CHLORIDE is probably combustible. | [Description]
Anti-infective (topical). Has been used as anthelmintic (Nematodes), as indicator for copper salts.
| [Description]
Crystal Violet is light sensitive. May react vigorously with strong oxidizing agents. May react exothermically with reducing agents to release gaseous hydrogen.
| [Chemical Properties]
dark green powder or crystals | [Chemical Properties]
This is a perennial, herbaceous plant native to mountainous areas of Europe; it may reach 0.5 to 1.0 m (2 to 3 ft) in height It has large, cylindrical roots (internally yellow); erect chalice; and fowers with a yellow corolla and peduncles It blooms from July to August The part used is the root of two-year-old plants The color of the rhizomes ranges from dark brown to light tan The color of roots appears to be related to its bitter principle content The dried product and its derivatives of gentian exhibit a very bitter favor. | [Occurrence]
Gentian is a fl owering perennial found in Europe and Asia. | [Uses]
As dye for wood, silk, paper; in inks; as biological stain. | [Definition]
ChEBI: Crystal violet is an organic chloride salt that is the monochloride salt of crystal violet cation. It has been used in creams for the topical treatment of bacterial and fungal infections, being effective against some Gram-positive bacteria (notably Staphylococcus species) and some pathogenic fungi (including Candida species) but use declined following reports of animal carcinogenicity. It has also been used for dying wood, silk, and paper, as well as a histological stain. It has a role as a histological dye, an antiseptic drug, an antibacterial agent, an antifungal agent and an anthelminthic drug. It contains a crystal violet cation. | [Brand name]
Genapax (Key); Gvs (Savage). | [Essential oil composition]
A number of bitter compounds present in gentian are primarily amarogentin (strongly bitter), gentiopricin
(approximately 1.5% in fresh root), swertiamarin and gentiopricroside. The leaves and flowers contain mainly xanthones.
Secoiridoids and flavonoids were also detected. In the phase of flowering, leaves are rich with compounds possessing C-glycoside
structures while O-glycoside structures accumulate mainly before flowering. | [Flammability and Explosibility]
Notclassified | [Biochem/physiol Actions]
Crystal violet can be used for DNA visualization in agarose gels. The dye is used only in the presence of high concentrations of DNA. Crystal violet is also used for the staining of bacteria in gram staining technique. It is also used for the staining of plant chromosomes. Crystal Violet also helps in colorimetric measurement of cell viability. | [Clinical Use]
Gentian violet is variously known as hexamethyl-p-rosanilinechloride, crystal violet, methyl violet, and methylrosanilinechloride. It occurs as a green powder or greenflakes with a metallic sheen. The compound is soluble inwater (1:35) and alcohol (1:10) but insoluble in nonpolar organicsolvents. Gentian violet is available in vaginal suppositoriesfor the treatment of yeast infections. It is also used asa 1% to 3% solution for the treatment of ringworm and yeastinfections. Gentian violet has also been used orally as an anthelminticfor strongyloidiasis (threadworm) and oxyuriasis. | [storage]
4°C, protect from light | [Properties and Applications]
|
TEST ITEMS
|
SPECIFICATION
|
|
APPEARANCE
|
DARK GREEN POWDER OR GRANULE
|
|
SHADE
|
BLUISH
|
|
HEAT RESISTANCE
|
200 °C min
|
|
DENSITY
|
1.07 g/cm
3
|
|
WATER FASTNESS
|
4-5
|
|
LIGHT FASTNESS
|
1-2
|
|
BLEACHABILITY (OXIDATIVE)
|
4
|
|
BLEACHABILITY (REDUCTIVE)
|
3
|
|
WATER SOLUBILITY AT
25 °C
|
16 g/L min
|
|
WATER INSOLUBLE
|
1.0% max
|
|
MOISTURE
|
3.0% max
|
|
TINTING STRENGTH
|
100-105 %
|
|
WEIGHT METAL TOTAL
|
50ppm max
|
| [Purification Methods]
Crystallise the dye from water (20mL/g), the crystals being separated from the chilled solution by centrifugation, then wash them with chilled EtOH (solubility is 1g in 10 mL of hot EtOH) and diethyl ether and dry under vacuum. It is soluble in CHCl3 but insoluble in Et2O. The carbinol is precipitated from an aqueous solution of the dye-hydrochloride, using excess NaOH, then dissolve in HCl and recrystallise it from water as the chloride [UV and kinetics: Turgeon & La Mer J Am Chem Soc 74 5988 1952]. The carbinol base has m 195o (needles from EtOH). The diphthalate (blue and turns red in H2O) crystallises from H2O, m 153-154o(dec at 185-187o)[Chamberlain & Dull J Am Chem Soc 50 3089 1928]. [Beilstein 13 H 233, 13 IV 2284.] |
| Questions and Answers (Q&A) | Back Directory | [General Description]
It belongs to triamino-triphenylmethane synthetic dyes; alkaline. It is an important dye used in bacterial Gram stain. The mixed reagent of crystal violet chloride and iodine is known as gentian violet.
| [Preparation]
It is manufactured through using N, N-dimethylaniline as raw materials, followed by condensation, addition, chlorination and other reactions. Alternatively, it can be synthesized through the reaction between Michler ketone and N, N-dimethylaniline reaction in the presence of phosphorus oxychloride, followed by azeotropic reaction with hydrochloric acid.
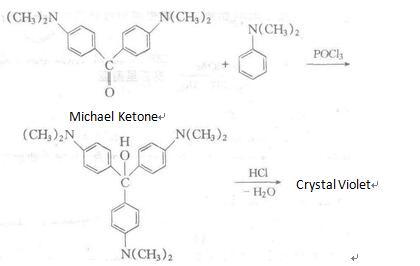 Recrystallization in hot water will generate compound containing nine crystal water molecules.
Recrystallization in hot water will generate compound containing nine crystal water molecules.
|
| Questions And Answer | Back Directory | [Physical and Chemical Properties]
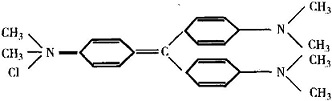
Crystal Violet is also known as "purple", "methyl violet", "gentian violet." It belongs to triphenylmethane type alkaline dyes. Scientific name:"hexamethyl chloride rose aniline." It appears as dark green powder with metallic luster. It is soluble in water, alcohol and chloroform, but insoluble in ether. Its solubility in water is 1.68% and its solubility in 95% ethanol is 13.87%. However, the solubility of its iodide in two solvents is only 0.035% and 1.78% respectively. Both its aqueous solution and alcohol solution are purple. The structural formula of its chloride is:
 | [Application]
Crystal Violet can be used as the dyes of silk, paper and acrylic as well as biological stains. It can be used for manufacturing paints and printing inks. It can also used as an acid-alkaline indicator with coloring range being from pH 0.5 (green) to 2.0 (blue), and developing reagent for colorimetric assays.
It can form a colored chelate with thallium in the hydrobromic acid medium, thus it can be used as thallium sensitivity reagent. It can also be used for the determination of other metal ions such as zinc, antimony, titanium, cadmium, tungsten, gold, mercury and so on. It can also be used as biological stains and non-aqueous titration acid-base indicator. In addition, it can also be used as antiseptic for inhibiting Staphylococcus aureus, Streptococcus and some fungi. Its 1% aqueous solution is commonly known as "purple syrup" for the prevention and treatment of skin and mucous membrane infections. Its enteric-coated tablets can be used as orally anti-pinworm medicine. |
|
|