| Identification | More | [Name]
2,6-Dichloropurine | [CAS]
5451-40-1 | [Synonyms]
2,6DCP
2,6-DICHLORO-9H-PURINE
2,6-DICHLOROPURINE
DCP
2,6-Dichloropurine.97%
2,6-dichloro-1H-purine
Dichloropurine
1H-Purine, 2,6-dichloro-
Cefepime Intermediate | [EINECS(EC#)]
226-681-6 | [Molecular Formula]
C5H2Cl2N4 | [MDL Number]
MFCD00077725 | [Molecular Weight]
189 | [MOL File]
5451-40-1.mol |
| Chemical Properties | Back Directory | [Appearance]
white to light yellow crystal powder | [Melting point ]
185-195 °C (dec.) (lit.) | [Boiling point ]
310.62°C (rough estimate) | [density ]
1.7265 (rough estimate) | [refractive index ]
1.6300 (estimate) | [storage temp. ]
2-8°C
| [solubility ]
soluble | [form ]
Crystalline Powder | [pka]
5.22±0.20(Predicted) | [color ]
White to yellow | [Water Solubility ]
soluble | [BRN ]
9354 | [InChIKey]
RMFWVOLULURGJI-UHFFFAOYSA-N | [CAS DataBase Reference]
5451-40-1(CAS DataBase Reference) |
| Safety Data | Back Directory | [Hazard Codes ]
Xn,Xi | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin .
R22:Harmful if swallowed. | [Safety Statements ]
S22:Do not breathe dust .
S24/25:Avoid contact with skin and eyes .
S37/39:Wear suitable gloves and eye/face protection .
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36:Wear suitable protective clothing . | [RIDADR ]
UN2811 | [WGK Germany ]
3
| [Hazard Note ]
Irritant | [HazardClass ]
IRRITANT | [HS Code ]
29339900 |
| Raw materials And Preparation Products | Back Directory | [Raw materials]
HYPOXANTHINE-1-OXIDE-->ADENINE N(1)-OXIDE MONOHYDRATE, 98-->1,5-Dihydropyrrolo[3,2-a]pyrimidine-2,4-dion-->Isoamyl nitrite-->Xanthine-->6-Chloroguanine-->Guanine | [Preparation Products]
2,6-Dichloro-7-methylpurine-->Cladribine-->7H-Purine, 2,6-dichloro-7-(1-methylethyl)--->2,6-dichloro-9-(1-methylethyl)-9H-purine-->9H-Purine, 2,6-dichloro-9-propyl- |
| Hazard Information | Back Directory | [Description]
2,6-Dichloropurine is an important pharmaceutical intermediate. It is widely used in the preparation of purine nucleosides and purine nucleotides[1].
| [Chemical Properties]
white to light yellow crystal powder | [Uses]
2,6-Dichloropurine is used in the synthesis of 2,6-diamino-substituted purine derivatives as potential cardiomyogenesis inducing agents. | [Uses]
Suzuki-Miyaura cross-coupling between halopurines and arylboronic acids in water-acetonitrile.1 | [Synthesis Reference(s)]
Journal of the American Chemical Society, 80, p. 404, 1958 DOI: 10.1021/ja01535a040 | [Synthesis]
2,6-Dichloropurine is prepared in two main ways:
(1) By chlorination of the purine ring structure, e.g., chlorination of xanthine (2,6-dihydroxypurine) with pyrophosphoryl chloride at high temperatures in sealed tubes in the presence of a phase-transfer catalyst or with phosphorus oxychloride under reflux, and chlorination of 6-chloropurine, hypoxanthine, or their N-oxides with phosphorus oxychloride, and chlorination with chlorine gas at low temperatures. Chlorination of 2,6-dithiopurine.
(2) The purine ring is constructed using barbituric acid derivatives or 2,4-dichloro-5,6-diaminopyridine as starting materials. However, both methods are not very suitable for industrial production.
The industrial preparation of 2,6-dichloropurine involves the direct chlorination of xanthine with phosphorus trichloride and a weakly nucleophilic organic base (e.g., amidine, guanidine base, or proton sponge)[1]. The reaction process is shown below:
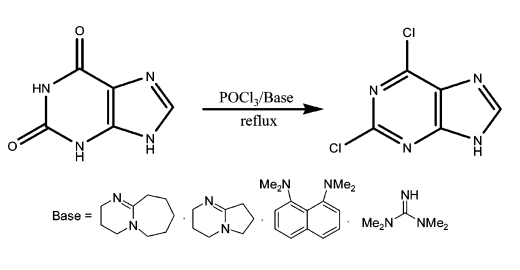
| [Purification Methods]
It can be recrystallised from 150 parts of boiling H2O and dried at 100o to constant weight. It is soluble in EtOAc. The HgCl2 salt separates from EtOH solution. UV: max 275nm ( 8.9K) at pH 1; and 280nm ( 8.5K) at pH 11 [Elion & Hitchings J Am Chem Soc 78 3508 1956, Schaeffer & Thomas J Am Chem Soc 80 3738 1958, Beaman & Robins J Appl Chem (London) 12 432 1962, Montgomery J Am Chem Soc 78 1928 1956]. [Beilstein 26 III/IV 1747.] | [References]
[1] QI ZENG. Facile and Practical Synthesis of 2,6-Dichloropurine[J]. Organic Process Research & Development, 2004, 8 6: 962-963. DOI:10.1021/op049878r. |
|
|