| Identification | More | [Name]
2,3,4,5-TETRAMETHYL-2-CYCLOPENTENONE | [CAS]
54458-61-6 | [Synonyms]
2,3,4,5-TETRAMETHYL-2-CYCLOPENTEN-1-ONE
2,3,4,5-TETRAMETHYL-2-CYCLOPENTENONE
2,3,4,5-TETRAMETHYLCYCLOPENT-2-ENONE
2,3,4,5-tetramethyl-2-cyclopentanone (cis+trans)
2,3,4,5-Tetramethyl-2-cyclopentenone (mixture of cis-and trans-isomers),
2,3,4,5-TETRAMETHYL-2-CYCLOPENTENONE, 95 %, MIXTURE OF CIS AND TRANS
2,3,4,5-tetramethyl-2-cyclopentenone, cis + trans
2,3,4,5-tetramethyl-2-cyclopentenone, mixture of cis and trans
2,3,4,5-Tetramethylcyclopent-2-enone 95%
2,3,4,5-Tetramethylcyclopenta-2-ene-1-one | [EINECS(EC#)]
611-148-6 | [Molecular Formula]
C9H14O | [MDL Number]
MFCD00010248 | [Molecular Weight]
138.21 | [MOL File]
54458-61-6.mol |
| Chemical Properties | Back Directory | [Boiling point ]
100 °C30 mm Hg(lit.)
| [density ]
0.927 g/mL at 20 °C(lit.)
| [refractive index ]
n20/D 1.476
| [Fp ]
164 °F
| [storage temp. ]
Keep in dark place,Sealed in dry,Room Temperature | [solubility ]
Chloroform, Methanol | [form ]
Oil | [color ]
Clear Colourless | [Specific Gravity]
0.917 | [Water Solubility ]
Not miscible with water. | [BRN ]
2324088 | [InChI]
InChI=1S/C9H14O/c1-5-6(2)8(4)9(10)7(5)3/h5,7H,1-4H3 | [InChIKey]
ARUAYSANQMCCEN-UHFFFAOYSA-N | [SMILES]
C1(=O)C(C)C(C)C(C)=C1C | [CAS DataBase Reference]
54458-61-6(CAS DataBase Reference) |
| Safety Data | Back Directory | [Safety Statements ]
S23:Do not breathe gas/fumes/vapor/spray (appropriate wording to be specified by the manufacturer) .
S24/25:Avoid contact with skin and eyes . | [WGK Germany ]
3
| [HS Code ]
29142990 |
| Hazard Information | Back Directory | [Uses]
2,3,4,5-Tetramethyl-2-cyclopentenone can be used as organic synthesis intermediates and pharmaceutical intermediates, mainly used in laboratory research and development processes and chemical production processes. | [Synthesis]
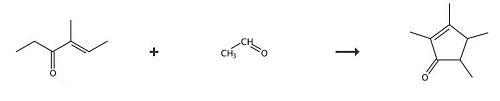
In a bottom round flask equipped with a mechanical stirrer, a dropping funnel and a reflux condenser was loaded 2000g (23.2 mol) of the starting ketone with 75% w/w of butylacetate as the solvent, 0.35 molar equivalents of anhydrous magnesium chloride and the aforementioned titanium catalytic solution containing 0.06 molar equivalents of the trichloropropoxytitanium complex. The resulting suspension was stirred vigorously and allowed to heat to 90°C. Then 2 molar equivalents of the acetaldehyde were added dropwise over 3h at 90°C. The reaction was continued for an additional hour and cooled to 40°C. The reaction mixture was hydrolysed with a 10% aqueous acetic acid solution and neutralised with a 20% aqueous potassium carbonate solution. The resulting organic phase was directly fractionated into a laboratory Sulzer packed column, to afford the title compound, as a mixture of isomers trans:cis = 85:15, in 27 % yield and the enone (II) (i.e. 4-methyl-4-hexen-3-one) in 31 % yield. mixture of isomers trans:cis = (B.p. = 70-80°C at P = 8 mbar); 4-methyl-4-hexen-3-one = (B.p. = 45-65°C at P = 8 mbar). 1H-NMR (isomer trans): 1.15 (d 3H); 1.19 (d 3H); 1.68 (s 3H); 1.88 (m 1H); 1.98 (s 3H); 2.25 (m 1H). 13C-NMR (isomer trans): 8.5; 14.6; 15.1 ; 17.7; 46.2; 48.4; 134.5; 171.6; 21 1.0. |
| Spectrum Detail | Back Directory | [Spectrum Detail]
2,3,4,5-TETRAMETHYL-2-CYCLOPENTENONE(54458-61-6)MS
2,3,4,5-TETRAMETHYL-2-CYCLOPENTENONE(54458-61-6)1HNMR
2,3,4,5-TETRAMETHYL-2-CYCLOPENTENONE(54458-61-6)IR1
2,3,4,5-TETRAMETHYL-2-CYCLOPENTENONE(54458-61-6)Raman
|
|
|