| Identification | Back Directory | [Name]
IODOSOBENZENE | [CAS]
536-80-1 | [Synonyms]
PhIO
536-80-1
iodoso-benzen
IODOSOBENZENE
iodosyl-benzen
Iodosobenzene>
Benzene, iodosyl-
Iodosobenzene,>95%
Phenyloxoiodine(III)
[Oxoiodo(III)]benzene
Phenyliodine(III) oxide
IODOSOBENZENE ISO 9001:2015 REACH
4-05-00-00692 (Beilstein Handbook Reference) | [EINECS(EC#)]
208-648-8 | [Molecular Formula]
C6H5IO | [MDL Number]
MFCD00039507 | [MOL File]
536-80-1.mol | [Molecular Weight]
220.01 |
| Chemical Properties | Back Directory | [Melting point ]
210°C (rough estimate) | [density ]
1.8665 (estimate) | [storage temp. ]
Freezer | [solubility ]
Methanol (Slightly), TFA (Slightly) | [Water Solubility ]
Slightly soluble in water | [form ]
powder to crystal | [color ]
White to Yellow to Green | [Merck ]
14,5044 | [InChIKey]
JYJVVHFRSFVEJM-UHFFFAOYSA-N | [EPA Substance Registry System]
Benzene, iodosyl- (536-80-1) |
| Hazard Information | Back Directory | [Chemical Properties]
Iodosobenzene is an amorphous yellow substance; it explodes at 210℃, decomposing with the evolution of iodine vapour, and dissolves in hot water and alcohol. If acids do not oxidise C6H5IO, they give saline compounds in which iodosobenzene appears as a basic oxide of a diatomic metal, C6H5I. Thus, for instance, when an acetic acid solution of iodosobenzene is treated with a solution of nitric acid, it gives large monoclinic crystals of a nitric acid salt having the composition C6H5(NO3)2 [like Ca(NO3)2). Iodosobenzene displaces iodine from potassium iodide (in a solution acidulated with acetic or hydrochloric acid)-ie, it acts with its oxygen like HClO. The action of peroxide of hydrogen, chromic acid, and other similar oxidising agents gives C6H5IO2, which is a neutral substance-i.e, is incapable of giving salts with acids.
Iodosobenzene is one of the very first oxidants and remains in use because it has excellent oxygen-transfer behavior and mechanistic cleanliness (Hill & Schardt, 1980; Rezaeifard et al., 2007; Po?towicz et al., 2006).
The Principles of Chemistry Volume 1 | [Uses]
Oxygen transfer reagent for stiochiometric or catalytic cross-functionalization of alkenes, alcohols, sulfides, and organometallo Compounds. | [Synthesis]
Iodosobenzene has been prepared by the action of sodium or potassium hydroxide solution on iodobenzene dichloride and by addition of water to the dichloride.
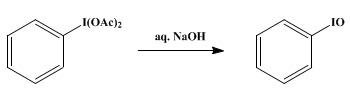
Iodosobenzene is prepared from iodobenzene.It is prepared by first oxidizing iodobenzene by peracetic acid. Hydrolysis of resulting diacetate affords "PhIO":
C6H5I + CH3CO3H + CH3CO2H → C6H5I(O2CCH3)2 + H2O
C6H5I(O2CCH3)2 + H2O → C6H5IO + 2CH3CO2H
http://orgsyn.org |
|
| Company Name: |
ABINTIO BIOSCIENCES
|
| Tel: |
+91-6302904517 +91-7386106841 |
| Website: |
www.abintiobiosciences.com |
| Company Name: |
Innovative Labs
|
| Tel: |
+91-9885794886 +91-9885794886 |
| Website: |
www.innovativelabsindia.com |
| Company Name: |
Riddhesh Pharmachem
|
| Tel: |
+91-9106014993 +91-9106014993 |
| Website: |
www.riddheshpharmachem.com |
|