| Identification | More | [Name]
Acetylsalicylic acid | [CAS]
50-78-2 | [Synonyms]
2-ACETOXYBENZOIC ACID
2-(ACETYLOXY)-BENZOIC ACID
ACETICYL
ACETOXYBENZOIC ACID
ACETYLSALICYLIC ACID
ACETYLSALICYLIC ACID IMPURITY D
ACETYSALICYLIC ACID
AKOS BBS-00003798
ALKENYL SUCCINIC ANHYDRIDES
ASA
ASPIRIN
O-ACETOXYBENZOIC ACID
O-ACETYLSALICYLIC ACID
2-(acetyloxy)-benzoicaci
2-Acetyloxybenzoesαure
2-acetyloxybenzoic
2-Carboxyphenyl acetate
A.S.A. Empirin
a.s.a.empirin
AC 5230 | [EINECS(EC#)]
200-064-1 | [Molecular Formula]
C9H8O4 | [MDL Number]
MFCD00002430 | [Molecular Weight]
180.16 | [MOL File]
50-78-2.mol |
| Chemical Properties | Back Directory | [Appearance]
Acetylsalicylic acid is a white crystalline solid with a slightly bitter taste. It is odorless but hydrolyzes in moist air to give an acetic acid odor | [Melting point ]
134-136 °C (lit.) | [Boiling point ]
272.96°C (rough estimate) | [density ]
1.35
| [refractive index ]
1.4500 (estimate) | [Fp ]
250 °C
| [storage temp. ]
Store at RT. | [solubility ]
H2O: 10 mg/mL at 37 °C
| [form ]
crystalline
| [color ]
white
| [pka]
3.5(at 25℃) | [Stability:]
Stable. Keep dry. Incompatible with strong oxidizing agents, strong bases, strong acids, various other compounds such as iodides, iron salts, quinine salts, etc. | [biological source]
synthetic | [Water Solubility ]
3.3 g/L (20 ºC) | [Usage]
Analgesic; antipyretic; anti-inflammatory; antithrombotic | [ε(extinction coefficient)]
190 at 298nm in aqueous base at 1mM
409 at 231nm in aqueous base at 1mM
466 at 230nm in aqueous acid at 1mM
68 at 278nm in aqueous acid at 1mM | [Merck ]
14,851 | [BRN ]
779271 | [Exposure limits]
ACGIH: TWA 5 mg/m3
NIOSH: TWA 5 mg/m3 | [BCS Class]
3 | [InChIKey]
BSYNRYMUTXBXSQ-UHFFFAOYSA-N | [LogP]
1.190 | [CAS DataBase Reference]
50-78-2(CAS DataBase Reference) | [NIST Chemistry Reference]
Benzoic acid, 2-(acetyloxy)-(50-78-2) | [EPA Substance Registry System]
50-78-2(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
Xn | [Risk Statements ]
R22:Harmful if swallowed.
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection . | [OEB]
B | [OEL]
TWA: 5 mg/m3 | [RIDADR ]
UN 1851 | [WGK Germany ]
1
| [RTECS ]
VO0700000
| [TSCA ]
Yes | [HazardClass ]
6.1 | [PackingGroup ]
III | [HS Code ]
29182200 | [Safety Profile]
Poison by ingestion, intraperitoneal, and possibly other routes. Human systemic effects by ingestion: acute pulmonary edema, body temperature increase, changes in kidney tubules, coma, constipation, dehydration, hematuria, hepatitis, nausea or vomiting, respiratory stimulation, somnolence, tinnitus, decreased urine volume. Implicated in aplastic anemia. A 10 gram dose to an adult may be fatal. A human teratogen. Human reproductive effects by ingestion and possibly other routes: menstrual cycle changes, parturition, various effects on newborn including Apgar score, developmental abnormalities of the cardlovascular and respiratory systems. Experimental animal reproductive effects. Human mutation data reported. An allergen; skin contact, inhalation, or ingestion can cause asthma, sneezing, irritation of eyes and nose, hves, and eczema. Combustible when exposed to heat or flame. When heated to decomposition it emits acrid smoke and fumes. | [Hazardous Substances Data]
50-78-2(Hazardous Substances Data) | [Toxicity]
LD50 orally in mice, rats: 1.1, 1.5 g/kg (Hart) |
| Hazard Information | Back Directory | [General Description]
Odorless white crystals or crystalline powder with a slightly bitter taste. | [Reactivity Profile]
The active ingredient in common aspirin. Incompatible with oxidizers and strong acids. Also incompatible with strong bases. May react with water or nucleophiles (e.g. amines and hydroxy groups). May also react with acetanilide, amidopyrine, phenazone, hexamine, iron salts, phenobarbitone sodium, quinine salts, potassium and sodium iodides, alkali hydroxides, carbonates, stearates and paracetanol. | [Air & Water Reactions]
Slowly hydrolyzes in moist air. Has been involved in dust cloud explosions. Water insoluble. Solution in water is acid to methyl red indicator. | [Hazard]
An allergen; may cause local bleeding espe-
cially of the gums; 10-g dose may be fatal. May
cause excessive biosynthesis of prostaglandins.
Dust dispersed in air is serious explosion risk. Skin
and eye irritant.
| [Potential Exposure]
Used as an over-the counter and proprietary pharmaceutical and veterinary drug. Those engagedin manufacture of aspirin or, more likely, in its consumption in widespread use as an analgesic, antipyretic, and antiinflammatory agent
| [Fire Hazard]
This chemical is combustible. | [First aid]
Move victim to fresh air. Call 911 or emergency medical service. Give artificial respiration if victim is not breathing. Do not use mouth-to-mouth method if victim ingested or inhaled the substance; give artificial respiration with the aid of a pocket mask equipped with a one-way valve or other proper respiratory medical device. Administer oxygen if breathing is difficult. Remove and isolate contaminated clothing and shoes. In the case of contact with substance, immediately flush skin or eyes with running water for at least 20 minutes. For minor skin contact, avoid spreading material on unaffected skin. Keep victim warm and quiet. Effects of exposure (inhalation, ingestion or skin contact) to substance may be delayed. Ensure that medical personnel are aware of the material(s) involved and take precautions to protect themselves. Medical observation is recommended for 24 to 48 hours after breathing overexposure, as pulmonary edema may be delayed. As first aid for pulmonary edema, a doctor or authorized paramedic may consider administering a drug or other inhalation therapy. | [Shipping]
UN2811 Toxic solids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required | [Incompatibilities]
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, carbonates, moisture. Dust dispersed in air is explosive | [Description]
Acetylsalicylic acid is a white crystalline powder commonly known by its common name as aspirin or ASA. Aspirin is the most widely used medication in the world. | [Chemical Properties]
Acetylsalicylic acid is a white crystalline solid with a slightly bitter taste. It is odorless but hydrolyzes in moist air to give an acetic acid odor | [Chemical Properties]
Aspirin (USAN), also known as acetyl salicylic acid (abbreviated ASA ) , is a salicylate drug, often used as an analgesic to relieve minor aches and pains, as an antipyretic to reduce fever, and as an anti - inflammatory medication. Aspirin may be effective at preventing certain types of cancer, particularly colorectal cancer.
The main undesirable side effects of aspirin taken by mouth are gastrointestinal ulcers, stomach bleeding, and tinnitus, especially in higher doses. In children and adolescents, aspirin is no longer indicated to control flu - like symptoms or the symptoms of chickenpox or other viral illnesses, because of the risk of Reye's syndrome.
Aspirin is part of a group of medications called non steroidal anti - inflammatory drugs (NSAIDs), but differs from most other NSAIDs in the mechanism of action. Though it, and others in its group called the salicylates, have similar effects (antipyretic, antiinflammatory, analgesic) to the other NSAIDs and inhibit the same enzyme cyclooxygenase, aspirin (but not the other salicylates) does so in an irreversible manner and, unlike others, affects more the COX-1 variant than the COX-2 variant of the enzyme.
Today, aspirin is one of the most widely used medications in the world, with an estimated 40,000 tonnes of it being consumed each year . In countries where Aspirin is a registered trademark owned by Bayer, the generic term is acetylsalicylic acid (ASA). | [Chemical Properties]
White Solid | [Waste Disposal]
May be flushed to sewer with large volumes of water. | [Physical properties]
Aspirin, an acetyl derivative of salicylic acid, is a white, crystalline, weakly acidic substance, with a melting point of 136 °C , and a boiling point of 140 °C .
Synthesis
The synthesis of aspirin is classified as an esterification reaction. Salicylic acid is treated with acetic anhydride, an acid derivative, causing a chemical reaction that turns salicylic acid's hydroxyl group into an ester group (R-OH → R-OCOCH3). This process yields aspirin and acetic acid, which is considered a byproduct of this reaction.
Polymorphism
Polymorphism, or the ability of a substance to form more than one crystal structure, is important in the development of pharmaceutical ingredients. Many drugs are receiving regulatory approval for only a single crystal form or polymorph. For a long time, only one crystal structure for aspirin was known. That aspirin might have a second crystalline form was suspected since the 1960s. The elusive second polymorph was first discovered by Vishweshwar and coworkers in 2005 , and fine structural details were given by Bond et al. . | [Originator]
Entab,Mayrand,US,1982 | [History]
The use of salicylic acid goes back thousands of years, and there are numerous accounts of the medicinal properties of plants from the Salix (willow) and Myrtaceae (Myrtle) families. Writings from ancient civilizations indicate the use of willow bark in Mesopotamia and myrtle leaves in Egypt as medicines existing several thousand years b.c.e. Hippocrates (460–377 b.c.e. ) and the ancient Greeks used powdered willow bark and leaves to reduce fever (antipyretic) and as a pain reliever (analgesic). Willow and oil of wintergreen was used as medications by native Americans.
The chemical responsible for the medicinal properties in willow and oil of wintergreen are forms of salicylates, a general name to describe compounds containing the general structure of salicylic acid. Willows (genus Salix) contain salicin and oil of wintergreen contains methyl salicylate. Although the use of willow bark and oil of wintergreen as an accepted antipyretic and analgesic has occurred for at least 2,000 years, by the 19th century medicines were starting to be synthesized in chemical laboratories. | [Definition]
ChEBI: A member of the class of benzoic acids that is salicylic acid in which the hydrogen that is attached to the phenolic hydroxy group has been replaced by an acetoxy group. A non-steroidal anti-inflammatory drug with moA cyclooxygenase inhibitor activity. | [Indications]
Aspirin is available as capsules, tablets, enteric-coated
tablets (Ecotrin), timed-release tablets (ZORprin),
buffered tablets (Ascriptin, Bufferin), and as rectal suppositories.
Sodium salicylate is available generically.
Other salicylates include choline salicylate (Arthropan),
choline magnesium trisalicylate (Trilisate), and magnesium
salicylate (Momentum). | [Manufacturing Process]
As described in US Patent 2,731,492, a glass-lined reactor of 1,500 gallons
capacity, fitted with a water-cooled reflux condenser, thermometers with
automatic temperature registers and an efficient agitator, is employed.
To start the process, a mother liquor is made by dissolving 1,532 kg of acetic
anhydride (15 mols) in 1,200 kg of toluene. To this mother liquor, add 1,382
kg of salicylic acid (10 mols), heat the reaction mixture under an efficient
reflux condenser, to 88-92°C and maintain within this temperature range for
20 hours.
The reaction mixture is now transferred to aluminum cooling tanks, and is
allowed to cool slowly, over a period of 3 to 4 days, to a terminal temperature
of 15-25°C (room temperature). The acetylsalicylic acid precipitates as large,
regular crystals. The mother liquor is now filtered or centrifuged from the
precipitated acetylsalicylic acid and the filter cake is pressed or centrifuged as
free of mother liquor as possible. The crystals are washed with distilled water
until completely free of acetic acid, pressed or centrifuged as dry as possible
and the filter cake is then dried in a current of warm air at a temperature of
60-70°C.
The filtrate from this first batch will comprise a solution of 180 to 270 kg of
unprecipitated acetylsalicylic acid (1.0 to 1.5 mols), 510 kg of acetic
anhydride (5.0 mols), 600 kg of acetic acid (10.0 mols) (obtained as a byproduct
in the acetylation step) and 1,200 kg of the diluent toluene. Into this
filtrate, at a temperature of 15° to 25°C, ketene gas is now passed through a
sparger tube or diffuser plate, with good agitation, until a weight increase of
420.5 kg of ketene (10 mols) occurs. The reaction mixture will now contain
180-270 kg of unprecipitated acetylsalicylic acid (1.0-1.5 mols) and 1,532 kg
of acetic anhydride (15 mols) in 1,200 kg of toluene. This mother liquor is
recycled to the first step of the process for reaction with another batch of
1,382 kg of salicylic acid. On recirculating the mother liquor, the yield of pure
acetylsalicylic acid is 1,780 to 1,795 kg per batch.
| [Brand name]
Aspirin;Compralgyl;Melabon;Rumicine;Salipran;Spalt;Tapal;Zorprin. | [Therapeutic Function]
Analgesic, Antipyretic, Antiinflammatory | [World Health Organization (WHO)]
Acetylsalicylic acid, a nonsteroidal anti-inflammatory, analgesic and
antipyretic agent, was introduced into medicine in 1899 and has since been widely
available in over-the-counter preparations. Recent studies carried out in the USA
have shown an association between acetylsalicylic acid consumption in children and
the development of Reye's syndrome (a rare condition characterized by a
combination of encephalopathy and liver disorder and usually preceded by an acute
viral illness, such as influenza, diarrhoea, or chickenpox). Many drug regulatory
authorities have acted to caution against the use of the drug in children and young
adults with febrile conditions. Even within this group the risk of exposure is remote
and has been estimated to be of the order of 1.5 per million. This warning also
concerns products containing other salicylates. The new indication of acetylsalicylic
acid - prophylaxis of myocardial infarction due to its antithrombotic effect - requires
loneterm use and may lead to serious adverse reactions, including cerebral
haemorrhage. Acetylsalicylic acid retains a valuable place in medicine and remains in
the WHO Model List of Essential Drugs. | [Biological Functions]
Aspirin is one of the most important NSAIDs because
it decreases pain at predominantly peripheral
sites with little cortical interaction and thus has few
CNS effects. The prototypical COX-2 inhibitors are
celecoxib (Celebrex) and its chemical cousin, rofecoxib
(Vioxx). In addition to a role in inflammatory processes,COX-2 seems to play a role in colon cancer and
Alzheimer’s disease, providing potential additional uses
for COX-2-selective drugs. | [Acquired resistance]
Aspirin is rapidly absorbed in the stomach and quickly degraded by plasma cholinesterases
(half-life, 15–20 min). A once-daily dose of 160 mg of aspirin, which is much lower than dosages
needed for its anti-inflammatory/analgesic actions, is sufficient to completely inactivate platelet
COX-1 irreversibly. Higher doses of aspirin only contribute to its side effects, especially
internal bleeding and upper gastrointestinal irritations. | [Biochem/physiol Actions]
Blocks the production of prostaglandins by inhibiting cyclooxygenase (prostaglandin H synthase), with greater selectivity toward the COX-1 isoform. The antithrombotic effect is due to the inhibition of COX-1 in platelets that blocks thromboxane production and platelet aggregation. Chemopreventive against colorectal and other solid tumors. | [Mechanism of action]
Discovery of the mechanism
In 1971, British pharmacologist John Robert Vane, then employed by the Royal College of Surgeons in London, showed aspirin suppressed the production of prostaglandins and thromboxanes.
Suppression of prostaglandins and thromboxanes
Aspirin's ability to suppress the production of prostaglandins and thromboxanes is due to its irreversible inactivation of the cyclo oxygenase (PTGS) enzyme required for prostaglandin and thromboxane synthesis. Aspirin acts as an acetylating agent where an acetyl group is covalently attached to a serine residue in the active site of the PTGS enzyme.
COX-1 and COX-2 inhibition
There are at least two different types of cyclooxygenase: COX-1 and COX-2. Aspirin irreversibly inhibits COX-1 and modifies the enzymatic activity of COX-2. COX-2 normally produces prostanoids, most of which are proinflammatory. Aspirin-modified PTGS2 produces lipoxins, most of which are anti-inflammatory.
Additional mechanisms
Aspirin has been shown to have at least three additional modes of action. It uncouples oxidative phosphorylation in cartilaginous (and hepatic) mitochondria, by diffusing from the inner membrane space as a proton carrier back into the mitochondrial matrix, where it ionizes once again to release protons . In short, aspirin buffers and transports the protons. When high doses of aspirin are given, it may actually cause fever, owing to the heat released from the electron transport chain, as opposed to the antipyretic action of aspirin seen with lower doses.
Hypothalamic - pituitary - adrenal activity
Aspirin, like other medications affecting prostaglandin synthesis, has profound effects on the pituitary gland, which indirectly affects a number of other hormones and physiological functions. | [Pharmacology]
Although aspirin itself is pharmacologically active, it
is rapidly hydrolyzed to salicylic acid after its absorption,
and it is the salicylate anion that accounts for most
of the anti-inflammatory activity of the drug. The superior
analgesic activity of aspirin compared with sodium
salicylate implies that aspirin has an intrinsic activity
that is not totally explainable by its conversion to salicylic
acid. Aspirin inhibits COX-1 to a much greater extent
than COX-2; sodium salicylate is more selective for
COX-1. This, combined with the ability of aspirin to
acetylate proteins, might account for some of the therapeutic
and toxicological differences between aspirin
and the other salicylates.
The binding of salicylic acid to plasma proteins
varies with its plasma concentrations. At serum salicylic
acid concentrations of less than 100 μg/mL, 90 to 95%
is protein bound; at 100 to 400 μg/mL, 70 to 85% is protein
bound; and at concentrations greater than 400
μg/mL, 20 to 60% is protein bound. The plasma concentration
of salicylate that is associated with antiinflammatory
activity (200–300 μg/mL) is about six
times that needed to produce analgesia. At these higher
concentrations, salicylate metabolism is reduced, resulting
in a longer half-life for the drug. This reaction is a
consequence of the saturable enzyme systems that metabolize
salicylates. The plasma half-life for salicylate
has been estimated to be 3 to 6 hours at the lower (analgesic)
dosage and 15 to 30 hours at the higher (antiinflammatory)
dosages.The rate of hydrolysis of aspirin
to salicylic acid is not dose limited, and no differences in
the absorption of aspirin have been observed between
arthritic patients and normal individuals. | [Pharmacology]
Salicylic acid is a weak acid, and very little of it is ionized in the stomach after oral administration. Acetylsalicylic acid is poorly soluble in the acidic conditions of the stomach, which can delay absorption of high doses for eight to 24 hours. The increased pH and larger surface area of the small intestine causes aspirin to be absorbed rapidly there, which in turn allows more of the salicylate to dissolve. Owing to the issue of solubility, however, aspirin is absorbed much more slowly during overdose, and plasma concentrations can continue to rise for up to 24 hours after ingestion. | [Clinical Use]
Aspirin is used in the treatment of a number of conditions, including fever, pain, rheumatic fever, and inflammatory diseases, such as rheumatoid arthritis, pericarditis, and Kawasaki disease.
Pain
Asprin 325 MG for pain In most cases, aspirin is considered inferior to ibuprofen for the alleviation of pain, because aspirin is more likely to cause gastrointestinal bleeding . Aspirin is generally ineffective for those pains caused by muscle cramps, bloating, gastric distension, or acute skin irritation.
Headache
Aspirin, either by itself or in combined formulation, effectively treats some types of headache, but its efficacy may be questionable for others.
Aspirin or other overthe- counter analgesics are widely recognized as effective for the treatment of tension headache. Aspirin, especially as a component of an acetaminophen/aspirin/caffeine formulation (e.g., Excedrin Migraine), is considered a first - line therapy in the treatment of migraine, and comparable to lower doses of sumatriptan.
Fever
Like its ability to control pain, aspirin's ability to control fever is due to its action on the prostaglandin system through its irreversible inhibition of COX .
Heart attacks and strokes
For a subset of the population, aspirin may help prevent heart attacks and strokes. In lower doses, aspirin has been known to prevent the progression of existing cardiovascular disease, and reduce the frequency of these events for those with a history of them . ( This is known as secondary prevention.)
Post-surgery
After percutaneous coronary interventions (PCIs), such as the placement of a coronary artery stent, a US Agency for Healthcare Research and Quality guideline recommends that aspirin be taken indefinitely.Frequently, aspirin is combined with an ADP receptor inhibitor, such as clopidogrel, prasugrel or ticagrelor to prevent blood clots. This is called dual anti-platelet therapy (DAPT). | [Side effects]
Contraindications
Aspirin should not be taken by people who are allergic to ibuprofen or naproxen , or who have salicylate intolerance[70][71] or a more generalized drug intolerance to NSAIDs, and caution should be exercised in those with asthma or NSAID - precipitated bronchospasm.
Gastrointestinal
Aspirin use has been shown to increase the risk of gastrointestinal bleeding . Although some enteric-coated formulations of aspirin are advertised as being "gentle to the stomach", in one study, enteric coating did not seem to reduce this risk. Combining aspirin with other NSAIDs has also been shown to further increase this risk.
Central effects
Large doses of salicylate, a metabolite of aspirin, have been proposed to cause tinnitus (ringing in the ears) based on experiments in rats, via the action on arachidonic acid and NMDA receptors cascade.
's syndrome
';s syndrome, a rare but severe illness characterized by acute encephalopathy and fatty liver, can occur when children or adolescents are given aspirin for a fever or other illnesses or infections. | [Side effects]
The most common adverse effects produced by the
salicylates are GI disturbances. Occult blood loss from
the GI tract, peptic ulceration, and rarely, severe GI
hemorrhage can occur. Because salicylic acid is highly
bound to plasma proteins, it may be displaced by other
highly protein-bound drugs such as oral anticoagulants,
sulfonylureas, phenytoin, penicillins, and sulfonamides.
The nonacetylated salicylates have greatly reduced
effects on blood loss and produce fewer adverse GI
effects. In addition, they may be somewhat kidney sparing.
Salicylates may provoke hypersensitivity reactions
and prolonged bleeding time in some individuals.
Tinnitus, hearing impairment, blurred vision, and lightheadedness
are indicators of toxic dosages. The use of
aspirin in conjunction with any other NSAID is not recommended
because of the lack of evidence that such
combinations increase efficacy and because of the increased
potential for an adverse reaction. Salicylates are
contraindicated in children with febrile viral illnesses
because of a possible increased risk of Reye’s syndrome. | [Synthesis]
Aspirin, acetylsalicylic acid (3.2.2), is synthesized by the acetylation of salicylic
acid (3.2.1) using acetic anhydride or acetyl chloride [60¨C63].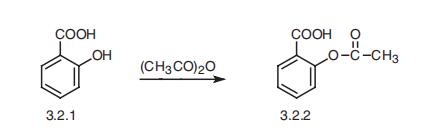
| [Drug interactions]
Potentially hazardous interactions with other drugs
ACE inhibitors and angiotensin-II antagonists:
antagonism of hypotensive effect, increased risk of
nephrotoxicity and hyperkalaemia.
Analgesics: avoid concomitant use of 2 or more
NSAIDs - increased side effects; avoid with
ketorolac - increased risk of side effects and
haemorrhage.
Antibacterials: possibly increased risk of convulsions
with quinolones.
Anticoagulants: effects of coumarins enhanced;
possibly increased risk of bleeding with edoxaban,
heparins and coumarins.
Antidepressants: increased risk of bleeding with
SSRIs and venlaflaxine.
Antidiabetic agents: effects of sulphonylureas
enhanced.
Antiepileptics: possibly increased phenytoin
concentration.
Antivirals: increased risk of haematological toxicity
with zidovudine; concentration possibly increased by
ritonavir.
Ciclosporin: may potentiate nephrotoxicity.
Cytotoxics: reduced excretion of methotrexate;
increased risk of bleeding with erlotinib. Diuretics: increased risk of nephrotoxicity;
antagonism of diuretic effect, hyperkalaemia with
potassium-sparing diuretics; increased risk of toxicity
of acetazolamide with high dose aspirin.
Lithium: excretion decreased.
Pentoxifylline: increased risk of bleeding.
Tacrolimus: increased risk of nephrotoxicity. | [Environmental Fate]
The toxicity of aspirin is multifactorial. Gastrointestinal
symptoms such as nausea, vomiting, and abdominal pain
occur as a result of both local gastric irritation and stimulation
of the medullary chemoreceptor trigger zone. Salicylates
directly stimulate the respiratory drive in the brain stem,
leading to hyperventilation and respiratory alkalosis. Anion
gap metabolic acidosis occurs from a buildup of organic acids
as well as the uncoupling of oxidative phosphorylation, which
results in an imbalance in ATP consumption and production,
resulting in a net buildup of hydrogen ions. Therefore, aspirin
often causes a mixed acid–base status. Furthermore, the
uncoupling of oxidative phosphorylation results in failure to
produce ATP despite increased oxygen utilization, which leads
to heat production and hyperthermia. Aspirin interferes with glucose metabolism and gluconeogenesis, and can cause
profound decreases in cerebrospinal fluid glucose concentrations
despite normal blood glucose concentrations. | [Metabolism]
After oral doses, absorption of non-ionised aspirin occurs
in the stomach and intestine. Some aspirin is hydrolysed
to salicylate in the gut wall. Once absorbed, aspirin is
rapidly converted to salicylate, but during the first 20
minutes after an oral dose aspirin is the main form of
the drug in the plasma. Both aspirin and salicylate have
pharmacological activity although only aspirin has an
anti-platelet effect. Salicylate is extensively bound to
plasma proteins and is rapidly distributed to all body
parts.
Salicylate is mainly eliminated by hepatic metabolism;
the metabolites include salicyluric acid, salicyl phenolic
glucuronide, salicylic acyl glucuronide, gentisic acid, and
gentisuric acid. The formation of the major metabolites,
salicyluric acid and salicyl phenolic glucuronide, is easily
saturated and follows Michaelis-Menten kinetics. As
a result, steady-state plasma-salicylate concentrations
increase disproportionately with dose. Salicylate is also
excreted unchanged in the urine; the amount excreted by
this route increases with increasing dose and also depends
on urinary pH, about 30% of a dose being excreted in
alkaline urine compared with 2% of a dose in acidic urine.
Renal excretion involves glomerular filtration, active renal
tubular secretion, and passive tubular reabsorption. | [storage]
Store at RT | [Purification Methods]
Crystallise aspirin twice from toluene, wash it with cyclohexane and dry it at 60o under vacuum for several hours [Davis & Hetzer J Res Nat Bur Stand 60 569 1958]. It has been recrystallised from isopropanol and from diethyl ether/pet ether (b 40-60o). It crystallises from EtOH (m 143-144o), *C6H6 (m 143o), hexane (m 115o and 128o), octane (m 121o), and has m 110o after sublimation. It has pK2 6 3.69(H2O), 4.15(20% aqueous EtOH), 4.47(30% aqueous EtOH) and 4.94(40% aqueous EtOH). It is an analgesic. [Beilstein 10 H 67, 10 II 41, 10 III 102, 10 IV 138.] | [Toxicity evaluation]
As in humans, the environmental fate of acetylsalicylic acid is
pH dependent. Above pH 5.5, acetylsalicylic acid will be the
predominant form seen. Anions generally do not volatilize or
undergo adsorption to the extent of their neutral counterparts.
Although information is limited, aspirin is considered readily
biodegradable and is ultimately mineralized to carbon dioxide
and water. |
| Questions And Answer | Back Directory | [What’s acetylsalicylic acid?]
Acetylsalicylic acid, also known as aspirin, is an analgesic-antipyretic medicine made by salicylic acid interacting with acetic anhydride. It is a white crystalline powder, odorless, stable in dry air. It will be slowly hydrolyzed to be salicylic acid and acetic acid in moist air, and aqueous solution has acidic reaction. Slightly soluble in water, soluble in ethanol, ethyl ether, chloroform, sodium hydroxide solution and sodium carbonate solution. Acetylsalicylic acid has antipyretic analgesic, anti-inflammatory and anti-rheumatism effect, that’s why it is often used for fever, headache, muscle pain, neuralgia, rheumatic fever, acute rheumatic arthritis, gout, etc.; also it has antiplatelet aggregation effect, and can be used for prevention of arterial thrombosis, atherosclerosis, transient cerebral ischemia and myocardial infarction; in addition, acetylsalicylic acid also can be used in the treatment of biliary tract roundworm disease and athlete's foot.
Pharmacological actions
Acetylsalicylic acid is one of the traditional antipyretic analgesics, as well as the role of platelet aggregation. Acetylsalicylic acid in the body has the characteristics of the antithrombotic, can reduce the formation of obstructive blood clots in surrounding arteries, and inhibit release of platelet response and endogenous ADP, 5-HT, etc., therefore to inhibit second phase other than the first phase of platelet aggregation. The mechanism of action of acetylsalicylic acid is to make platelets cyclooxygenase acetylation, thus inhibiting the formation of ring peroxide, and TXA2 formation is also reduced as well. At the mean time make the platelet membrane protein acetylation, and inhibit platelet membrane enzyme, which helps to inhibit platelet function. As the cyclooxygenase is inhibited, it impacts blood vessel wall synthesized to be PGI2, the platelet TXA2 synthetic enzymes also to be inhibited; so it would impact formation of both TXA2 and PGI2 when it is large doses. Suitable for ischemic heart disease, after percutaneous transluminal coronary angioplasty or coronary artery bypass grafting, prevent transient ischemic stroke, myocardial infarction and reduce the incidence of arrhythmia.
The above information is edited by the Chemicalbook He Liao Pu. | [Chemical property]
This product is a white crystalline, with melting point 138~140 ℃, insoluble in water, soluble in alcohol, ether, etc.
| [Uses]
1.Acetylsalicylic acid is the raw material for rodenticide intermediates 4-hydroxycoumarin.
2.Used to make outdoor structural members and equipment parts exposed in highlights, such as the automobile body, agricultural machinery parts, meters and electric lamps, road marking, etc.
3.It is the earliest applied, the most popular and the most common antipyretic analgesics anti-rheumatism medicine, has aspects of pharmacological effects as antipyretic-analgesic and anti-inflammatory, anti-platelet aggregation and works quickly and effectively. Overdosage can be easily diagnosed and treated, with rare allergic reactions. Often used to cold fever, headache, neuralgia, joint ache, muscle pain, rheumatic fever, acute wet sex arthritis, rheumatoid arthritis and toothache, etc. Listed in National Essential Medicine List. Acetylsalicylic acid also works as an intermediate of other medicines.
| [How Aspirin is used in Ischemic Stroke therapy]
In the therapy of Ischemic Stroke, 50 to 325 mg/day started between 24 and 48 hours after completion of alteplase has also been shown to reduce long-term death and disability.
Aspirin, clopidogrel, and extended-release dipyridamole plus aspirin are all considered first-line antiplatelet agents. The combination of aspirin and clopidogrel can only be recommended in patients with ischemic stroke and a recent history of myocardial infarction or coronary stent placement and then only with ultra-low-dose aspirin to minimize bleeding risk.
| [Production method]
Acetylsalicylic acid: add acetic anhydride (feeding ratio is 0.7889 times of the total salicylic acid) in reaction vessel, and then add two thirds of salicylic acid, stir it and temperature rises. React 40-60min in 81-82℃. Cool it to 81-82 ℃ and keep the temperature for 2h. When free salicylic acid is qualified, cool it to 13 ℃, precipitation crystallization, rejection filter, dry it in 65-70℃ air flow, then we get acetylsalicylic acid. | [Category]
Toxic substance | [Toxicity grading]
High toxic
| [Acute toxicity]
Oral-rat LD50: 200 mg/kg; Oral-mice LD50: 250 mg/kg
| [Flammable hazardous characteristics]
Flammable in fire; irritant gas would be decomposed out when heated.
| [Handling and storage characteristics]
Warehouse needs to be ventiIative and dry with low temperature; separate it from oxidant and food additives.
| [Fire extinguishing agent]
Water fog, foam, carbon dioxide, sandy soil.
| [Occupational standard]
TWA 5 mg/m3
|
|
|