| Identification | More | [Name]
Isotretinoin | [CAS]
4759-48-2 | [Synonyms]
13-CIS-RETINOIC ACID
13-cis-vitamin a acid
3,7-dimethyl-9-(2,6,6-trimethyl-1-cyclohexen-1-yl)-2-cis-4-trans-6-trans-8-trans-nonatetraenoic acid
3,7-DIMETHYL-9-(2,6,6-TRIMETHYL-1-CYCLOHEXEN-1-YL)-2Z,4E,6E,8E-NONATETRANENOIC ACID
ISOTRETINOIN
(L)-ISOTRETINOIN
RETINOIC ACID, 13-CIS-
13-cis-ra
13-cis-retinoicaci
13-ra
accutane
Isoretinoin
isotrex
neovitaminaacid
ro-4-3780
ro4-3780
roaccutane
teriosal
Lsotretinoin
ISOTRETINOIN, USP STANDARD | [EINECS(EC#)]
225-296-0 | [Molecular Formula]
C20H28O2 | [MDL Number]
MFCD00079542 | [Molecular Weight]
300.44 | [MOL File]
4759-48-2.mol |
| Chemical Properties | Back Directory | [Appearance]
Yellow-Orange Crystalline Powder | [Melting point ]
172-175 °C (lit.) | [Boiling point ]
381.66°C (rough estimate) | [density ]
1.0597 (rough estimate) | [refractive index ]
1.4800 (estimate) | [storage temp. ]
−20°C
| [solubility ]
Practically insoluble in water, soluble in methylene chloride, slightly soluble in ethanol (96 per cent). It is sensitive to air, heat and light, especially in solution. Carry out all operations as rapidly as possible and avoid exposure to actinic light; use freshly prepared solutions. | [form ]
Fine Crystalline Powder | [pka]
4.76±0.33(Predicted) | [color ]
Yellow-orange to orange | [Stability:]
Stable, but probably air and light sensitive. Combustible. Incompatible with strong oxidizing agents. | [biological source]
synthetic | [Water Solubility ]
insoluble | [Usage]
Used as a treatment for severe acne. Presently being studied in conjuction with the treatment of photoaged skin. | [λmax]
354nm(EtOH)(lit.) | [Merck ]
14,5228 | [InChIKey]
SHGAZHPCJJPHSC-YCNIQYBTSA-N | [Uses]
isotretinoin is a retinoid derivative with improved bioavailability and percutaneous absorption for acne treatment products. | [CAS DataBase Reference]
4759-48-2(CAS DataBase Reference) | [EPA Substance Registry System]
4759-48-2(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
T | [Risk Statements ]
R61:May cause harm to the unborn child.
R36/37/38:Irritating to eyes, respiratory system and skin .
R20/21/22:Harmful by inhalation, in contact with skin and if swallowed . | [Safety Statements ]
S53:Avoid exposure-obtain special instruction before use .
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) .
S37/39:Wear suitable gloves and eye/face protection . | [WGK Germany ]
3
| [RTECS ]
VH6440000
| [TSCA ]
Yes | [HS Code ]
29362100 | [Safety Profile]
Poison by
intraperitoneal route. Moderately toxic by
ingestion. A human teratogen by ingestion
with fetal developmental abnormalities of
the skin and appendages and other postnatal
effects. Human reproductive effects. Human
systemic effects: decreased immune
response, darrhea, hypermouhty, irritative
dermatitis, sweating. Human mutation data
reported. An experimental teratogen. Other
experimental reproductive effects. When
heated to decomposition it emits acrid
smoke and irritating fumes. | [Hazardous Substances Data]
4759-48-2(Hazardous Substances Data) | [Toxicity]
LD50 (20 day) in mice, rats (mg/kg): 904, 901 i.p.; 3389, >4000 orally (Kamm) |
| Raw materials And Preparation Products | Back Directory | [Raw materials]
Triphenylphosphine-->Ionone-->Ethylmagnesium chloride-->Palladium nitrate-->n-Butyllithium-->Diisopropylamine-->3-Methyl-5-(2,6,6-trimethyl-1-cyclohexen-1-yl)-2,4-pentadienal-->METHYL 3,4,5-TRIFLUOROBENZENECARBOXYLATE-->Methyl 3-methyl-2-butenoate-->Retinoic acid-->Iodomethane | [Preparation Products]
methyl (2Z,4E,6Z,8Z)-3,7-dimethyl-9-(2,6,6-trimethyl-1-cyclohexenyl)no na-2,4,6,8-tetraenoate |
| Hazard Information | Back Directory | [General Description]
Yellow-orange to orange crystalline powder; orange-brown chunky solid. | [Reactivity Profile]
An organic acid and unsaturated aliphatic hydrocarbon. Carboxylic acids donate hydrogen ions if a base is present to accept them. They react in this way with all bases, both organic (for example, the amines) and inorganic. Their reactions with bases, called "neutralizations", are accompanied by the evolution of substantial amounts of heat. Neutralization between an acid and a base produces water plus a salt. Insoluble carboxylic acids react with solutions of cyanides to cause the release of gaseous hydrogen cyanide. Flammable and/or toxic gases and heat are generated by the reaction of carboxylic acids with diazo compounds, dithiocarbamates, isocyanates, mercaptans, nitrides, and sulfides. Carboxylic acids, especially in aqueous solution, also react with sulfites, nitrites, thiosulfates (to give H2S and SO3), dithionites (SO2), to generate flammable and/or toxic gases and heat. Their reaction with carbonates and bicarbonates generates a harmless gas (carbon dioxide) but still heat. Like other organic compounds, carboxylic acids can be oxidized by strong oxidizing agents and reduced by strong reducing agents. These reactions generate heat. A wide variety of products is possible. Like other acids, carboxylic acids may initiate polymerization reactions; like other acids, they often catalyze (increase the rate of) chemical reactions. | [Air & Water Reactions]
Insoluble in water. | [Fire Hazard]
Flash point data for this material are not available; however, CIS-RETINOIC ACID is probably combustible. | [Description]
The original patent for Accutane expired in 2002. Held by
Hoffmann-LaRoche until that time, it is perhaps now better
known by its common name, isotretinoin, although over 100
trade names for the compound now exist. Isotretinoin was
originally developed to treat cystic acne, and today this is still
its primary use despite several more modern applications of
the drug, including a treatment for pancreatic and brain
cancers.
Isotretinoin is the 9-cis isomer of retinoic acid, a close
relative of retinol, or vitamin A. First shown to be an effective
treatment for acne in 1982, its development stemmed from
advances in knowledge of the effects of vitamin A to reduce or
eliminate sebum production. Since that time, however, several
instances of deleterious effects became well known, most
notably birth defects arising from the use of isotretinoin.
Accutane was removed from distribution by Roche in 2009
after several lawsuits had been filed alleging damages due to
side effects, especially in young adult males due to inflammatory
bowel disease (IBD). | [Chemical Properties]
Yellow or orange crystalline powder or crystal, insoluble in water, slightly soluble in ethanol, very slightly soluble in ether, soluble in chloroform. | [Originator]
Accutane,Roche | [Definition]
ChEBI: A retinoic acid that is all-trans-retinoic acid in which the double bond which is alpha,beta- to the carboxy group is isomerised to Z configuration. A synthetic retinoid, it is used for th
treatment of severe cases of acne and other skin diseases. | [Indications]
Isotretinoin (Accutane) alters keratinization in the
acroinfundibulum of sebaceous glands and shrinks
them, thereby reducing sebum excretion and comedogenesis.
These features underlie its usefulness in acne
vulgaris, since sebum secretion is a hallmark of acneprone
skin. Furthermore, the drug has antiinflammatory
activity. | [Preparation]
Preparation of isotretinoin in a single step from β-ionone and ethyl chloride are first reacted together after which the product is further reacted with triphenylphosphine to obtain Triphenyl salt. The Triphenyl salt is further reacted with cyclopentenone derivative to produce isotretinoin and its 8Z isomer. separate out the 8Z isomer and convert it to isotretinoin through isomerization with the help of nitric acid. | [Manufacturing Process]
Under an atmosphere of nitrogen, a solution of n-butyl lithium in hexane (321
ml, 15%) was added to a solution of diisopropylamine (48.6 g, 0.48 mole) in
tetrahydrofuran (1000 ml) at -30°C and the mixture was stirred for one hour.
The reaction mixture was then cooled to -72°C and methyl 3,3-dimethyl
acrylate (55 g, 0.48 mole) was added to it. Stirring was continued at -65° to -
75°C for 30 min. To the resulting mixture, a solution of β-ionylidene
acetaldehyde (100 g, 0.458 mole, 9-trans content: 80%) was added and the
reaction mixture was stirred at -65° to -75°C for 1 h. The reaction mixture
was then warmed to 40°C and stirred at this temperature for 3 h. Solvent was
removed under vacuum and the reaction mixture was diluted with water (700
ml) and methanol (300 ml). Activated charcoal (4 g) was then added and the
mixture was refluxed for 30 min. The heterogeneous mixture was filtered
through hyflo and the hyflo bed was washed with methanol (300 ml) and
water (150 ml). The aqueous methanolic layer was then extracted with
hexanes (2 x 500 ml) and acidified with 10% sulfuric acid to pH 2.80.5. The
desired product was then extracted with dichloromethane (2 x 500 ml). The
combined dichloromethane layer was washed with water (2 x 300 ml) and
concentrated in vacuo to afford the desired isotretinoin. Crystallization from
methanol (200 ml) afforded isotretinoin (44 g) in greater than 99% HPLC
purity. | [Brand name]
Accutane (Roche); Amnesteem
(Genpharm); Claravis (Barr); Sotret (Ranbaxy);Accutane roche;Apsor;Isotretinoin;Neovamin a acid;Neovitamin a acid;Ro 4-3780;Roacutan. | [Therapeutic Function]
Antiacne, Keratolytic | [World Health Organization (WHO)]
Isotretinoin, a retinol derivative, was introduced in 1982 exclusively for the treatment of severe acne. Its use in pregnant women has
resulted in major fetal abnormalities. The manufacturer's information emphasizes
that the drug is teratogenic and must not be given to women who are pregnant,
and that contraceptive measures must be maintained for at least four weeks after
discontinuation of treatment. In some countries, blood banks are advised not to
accept as donors persons who have taken isotretinoin within the previous four
weeks. See also under retinol (vitamin A). | [Biochem/physiol Actions]
13-cis-Retinoic acid (RA) has anti-inflammatory and anti-tumor action. The action of RA is mediated through RAR-β and RAR-α receptors. RA attenuates iNOS expression and activity in cytokine-stimulated murine mesangial cells. It induces mitochondrial membrane permeability transition, observed as swelling and as a decrease in membrane potential, and stimulates the release of cytochrome c implicating mechanisms through the apoptosis pathway. These activities are reversed by EGTA and cyclosporin A. RA also increases MMP-1 protein expression partially via increased transcription. | [Mechanism of action]
Isotretinoin is rapidly absorbed orally, with peak
blood concentrations 3 hours after ingestion. It is not
stored in tissue, and the elimination half-life is 10 to 20
hours, either after a single dose or during chronic therapy. | [Clinical Use]
Isotretinoin is most useful for the treatment of severe
recalcitrant nodular acne vulgaris. It may also be helpful in other disorders of keratinization, but it is
not useful for psoriasis. High doses of isotretinoin
(2mg/kg/day) are effective as cancer chemoprevention
agents to reduce the frequency of cutaneous malignancies
in patients at increased risk, such as those with xeroderma
pigmentosum, an inherited disorder in which
DNA repair is deficient, or in immunosuppressed patients. | [Side effects]
Isotretinoin is teratogenic to humans and should not be administered to pregnant women or women contemplating pregnancy. Concomitant use of isotretinoin with drugs of the tetracycline class increases the incidence of Pseudotumor cerebri. There have been recent reports of an increased risk of depression, suicide, and suicide attempts in individuals taking isotretinoin, but the causality has not been absolutely proved.
Isotretinoin, like many retinoids, can lead to increase in serum aminotransferase levels, but, unlike acitretin and etretinate, isotretinoin has not been clearly implicated in cases of clinically apparent acute liver injury with jaundice. | [Veterinary Drugs and Treatments]
Isotretinoin may be useful in treating a variety of dermatologicrelated
conditions,
including canine lamellar ichthyosis, cutaneus
T-cell lymphoma, intracutaneous cornifying epitheliomas,
multiple
epidermal inclusion cysts, comedo syndrome in Schnauzers,
and sebaceous adenitis seen in standard poodles.
Because of the concerns of teratogenic effects in humans, availability
to veterinarians may be restricted by the manufacturers and
drug distributors; obtaining the medication for veterinary patients
may be difficult. | [Drug interactions]
Potentially hazardous interactions with other drugs Antibacterials: possible increased risk of benign intracranial hypertension with tetracyclines - avoid. Antifungals: possible increased risk of toxicity with fluconazole, ketoconazole and voriconazole. Vitamins: increased risk of hypervitaminosis with vitamin A. | [Environmental Fate]
In primates (including humans), isotretinoin (Accutane) is
metabolized to a more active form, 13-cis-4-oxo-retinoic acid,
which is able to move through the placental membrane. On
its own, however, Accutane (isotretinoin) is not particularly
motile across the placental barrier, and perhaps most interestingly
tends not to bind to cellular retinoid-binding proteins
or nuclear receptors. The rapid isomerization to the all-trans
isomer, the oxidation of Accutane (isotretinoin) to 13-cis-4-
oxo-retinoic acid, and the relatively high circulation times of
these compounds may be important in explaining the teratogenic
toxicity of Accutane (isotretinoin).
Some studies have more fully explored the metabolic
products of isotretinoin. For example, isotretinoin can be
metabolized in the liver by the cytochrome P450 microsomal
enzyme system – more specifically the CYP2C8, CYP2C,
CYP3A4, and CYP2B6 isoenzymes. The metabolites produced
are numerous, including retinoic acid (tretinoin), 4-oxo-isotretinoin,
and 4-oxo-retinoic acid (4-oxo-tretinoin). This relatively
large array of retinoid metabolites may produce a variety
of effects, most notably due to their higher potency as retinoids
compared to the parent compound (isotretinoin).
It is possible that these additional metabolites are capable
of binding to a variety of retinoid receptors in order to alter
gene expression and further transcription or transrepression in
protein synthesis, which may be responsible for the toxic effects
of isotretinoin. | [Toxicity evaluation]
Direct studies focused on the environmental fate of Accutane
(isotretinoin) are rare in the literature. The pure compound is
insoluble in water, and highly lipophilic. Powders do not
aerosolize readily, and volatilization is extremely low. Isotretinoin
released into the environment would not be expected
to have high mobility in water or soil, and will most likely
become deposited in organic materials. Bioaccumulation is
possible, but isotretinoin is readily oxidized to form other
retinoids or metabolites that are expected to be mitigated via
natural biological pathways. |
| Questions And Answer | Back Directory | [Pharmacological effects]
Isotretinoin belongs to the first generation of vitamin A acid. It is a stereoisomer of all-trans vitamin A acid. Oral administration has an anti-oil effect which has special effect on severe acne. The mechanism is as follows:
① it can reduce sebaceous gland and inhibit the activity of sebaceous gland, reducing the effect of secretion of the sebaceous glands, thus inhibiting the growth of Propionibacterium which relies on lipid environment.
② it has an anti-keratosis effect, can inhibit the excess proliferation of epidermal and promote its differentiation. It also reduces the keratosis of hair follicles and the catheter of sebaceous glands.
③ it can also affect the function of lymphocytes and monocytes, and inhibit the chemotaxis of neutrophil, and thus having anti-inflammatory activity.
④ it can exert its therapeutic effect by selectively binding Vitamin A acid nuclear receptors. It can inhibit the proliferation of acne rod bacilli. Moreover it has effects of immunity stimulation at low doses, but has immunity suppression effect at high doses.
⑤ It can inhibit the biogenesis of collagenase and gelatinase in the skin, and can also inhibit the activity of ornithine decarboxylase. Owing to the major role of ornithine decarboxylase in inducing skin mutations, so isotretinoin can also suppress tumorigenesis.
⑥ when subjects to topical administration, it has a similar mechanism of action as vitamin A acid which induces the proliferation of epidermal cell, promotes the differentiation of epidermal cell granular layer into the stratum corneum. It can also regulate the horny plug occurring during some abnormal cornification processes of Hair follicle sebaceous epithelium, and thus playing a therapeutic role.
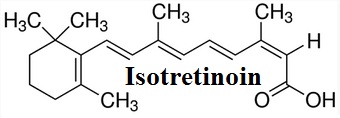
Figure 1 Structure formula of Isotretinoin
The above information is edited by the Chemicalbook of Dai Xiongfeng.
| [Chemical Properties]
It is orange-red flake crystals obtained from isopropanol, melting point 174~175°C. UV maximal absorption: 354nm (ε39800). Acute toxicity LD50 (20 days) mice, rat (mg/kg): 904,901 intraperitoneal injections; 3389> 4000 oral administration. | [Physical and Chemical Properties]
Yellow or orange crystalline powder or crystal, insoluble in water, slightly soluble in ethanol, very slightly soluble in ether, soluble in chloroform.
| [Pharmacokinetics]
This product is rapidly absorbed after oral administration. The plasma concentration reaches peak within 2 to 4 hours. However, oral administration has a low bioavailability which can be boosted by postprandial medication. It has a over 99% binding rate to the plasma protein. It is mainly metabolized in the liver or intestine wall. It enters into the enterohepatic circulation in the form of prototype and metabolites. Prototype drug without any changes is excreted from the feces while metabolite is excreted from the urine. The half life of elimination lasts 10 to 20 hours.
| [Uses]
Isotretinoin is a vitamin A class drugs that It can rapidly and strongly inhibit the cell proliferation and differentiation process of skin glands cells. It has a high effect on severe nodular cystic acne. This product has a high gastrointestinal absorption, but invalid for topical administration. It is used for treating severe acnes which cannot be treated by other drugs. It also has some effects on treating cystic acne, the party of acne, rosacea, ichthyosis, follicular keratosis and pityriasis red hair and other skin diseases. | [Indications]
1. Isotretinoin should be the primary choice for treatment of refractory acne, especially for severe nodular, cystic, inflammatory acne patients to whom conventional therapy is ineffective.
2. Generalized Plana or verrucous epidermal dysplasia;
3. Skin disease of abnormal cornification;
4. Cutaneous lupus erythematosus;
5. lichen planus, atrophic lichen sclerosis.
| [Clinical application]
Isotretinoin is an effective drug against acne. It takes effect on four processes of acne procedures, preventing the formation of acne lesions: ① inhibiting sebaceous secretion; ② inhibit the growth of acne bacillus; ③ inhibiting sebaceous hyperplasia; ④ anti-inflammatory effect. Isotretinoin has no affinity to Vitamin A acid receptors. Its exact mechanism of action is not very clear. Its anti-acne role may be related to that it or its metabolites can inhibit the proliferation and differentiation of sebaceous glands. It may also reduce the volume of the sebaceous glands, reducing the skin's DHT, down-regulate the expression of skin androgen receptor. Other possible mechanism includes reducing the hair follicle keratosis, antibacterial, anti-inflammatory effects and so on. In the 1980s, Isotretinoin is only for treating severe nodular cystic acne. Isotretinoin is now also used for treating some less-seriously acne where those related patients are often insensitive to conventional therapy, especially some patients with long-term use of antibiotics due to that acne bacillus have evolved multiple drug (such as erythromycin, tetracycline) resistance. It may also be used for some acne patients prone to scar.
Isotretinoin is usually applied with a relative high dose at the beginning in treatment of acne, such as 1 mg/(kg ? d). However, most patients follow this poorly due to adverse reactions of drugs. It was later found that 0.5 mg/(kg ? d) can also give similar therapeutic effect. In order to achieve the accumulation of appropriate amount, drug usage can be prolonged. The calculation method of accumulation of isotretinoin is using total amount of applied drugs to divide the patient body weight (mg/kg). In general, cumulative dose of 120mg/kg will effectively reduce the possibility of disease relapse. However, the maximum cumulative amount should no exceed 150mg/kg. Owing to the certain residence time of the drug in the body, the symptoms of some patients still keep going well even after stopping taking drugs, Therefore, it is not unnecessary to maintaining therapy until all lesions subsided. About one-third of patients required re-treatment (due to the persistent or recurrent disease). There are two cases of resistance to isotretinoin: closed comedones and small cystic acne. During the first 1 to 2 weeks of treatment, some patients will suffer an increasing skin lesion. Some cystic acne becomes pyogenic granuloma-like lesions. This is due to that taking isotretinoin does harm to the normal skin barrier function, leading to increased colonization of staphylococcus aureus. If some women exhibit resistance to isotretinoin therapy or suffer recurrent disease, we should observe whether hairy happens and menstrual cycle is normal. Also check the ovaries and adrenal function. Both adrenal dysfunction and high expression level of 5α-reductase can cause this issue.
| [Toxicity]
(1) Adverse reactions of skin and mucous membranes: This is the most common adverse reaction of orally administration of isotretinoin. The higher dose of oral administration is, the higher the incidences of adverse reactions happen. Cheilitis is the mostly common with a 100% incidence, exhibiting mucosa lips dry, chapped, peeling, bleeding, which is similar to exfoliative dermatitis; Nearly 30% to 50% of patients suffer drying nasal mucosa, bleeding, reversible hair loss, dry and itching skin, dry eye. This happens especially in patients with allergies and xerosis. Those patients who wear contact lenses should not use this drug. Or we should not wear contact lenses during taking it.
(2) Teratogenicity: Animal experiments show that oral administration of isotretinoin can cause malformations, miscarriage and stillbirth. Administration during the early phase of fetal organ formation can lead to the abnormal development of the central nervous system and cardiovascular system. Administration in the later phase of pregnancy can cause fetal limb shortage and defect on urinary system.
(3) Effect on the bone: Long-term application of isotretinoin can cause bone hypertrophy, tendon ligament calcification, osteoporosis, epiphyseal atresia, thus badly affecting the growth of children and adolescents. Bone hypertrophy and tendon ligament calcification are the most common. The incidence is related to the drug dosage and duration. When treated with 1~2mg dose per day per kilogram of body weight and a4 to 5 months duration of oral isotretinoin, about 10% of patients can be detected for bone hypertrophy.
(4) Effect on mental activity: people suffer from acne often mentally more depressed or prone to depression. Taking isotretinoin will boost this tendency. It has been reported since 1982 (when isotretinoin first entered into market) to May 2000, the US Food and Drug Administration (FDA) has reported 431 cases of mental disorders after taking isotretinoin, wherein 37 patients commit suicide, 110 patient got depression or suicidal thoughts or suicide for hospitalized, 284 patients with depression but not hospitalized. Effect of oral administration of isotretinoin on mental activity is related to the duration of the drugs; stopping taking drugs or psychiatric treatment can alleviate the symptoms. However, symptoms can reoccur, and even deteriorate after re-treatment again or worse.
(5) Laboratory abnormalities: oral administration of isotretinoin can cause elevated level of triglycerides and cholesterol in serum.
| [Drug Interactions]
1. Combination with warfarin can enhance the latter one’s effect.
2. Combination together with Avi A ester, vitamin A acetate or Vitamin A acid can increase the incidence and severity of adverse reactions.
3. Combination with methotrexate can increase the blood concentration of the latter one, and thus increasing the damage to the liver.
4. Combination with tetracycline can lead to brain and cause pseudo-tumor in brain and cause benigh hypertension. Clinical manifestations of hypertension are accompanied by headache, dizziness and visual disturbances.
5. Combination with carbamazepine results in decreased plasma concentrations of the latter one.
6. Combination with light-sensitive drugs increases the light-sensitive effect.
| [Contraindications]
1. Pregnant women, lactation, patients with severe liver and kidney dysfunction.
2. Take with caution for patients of hyperlipidemia, diabetes, severe osteoporosis.
3. Avoid apply together with vitamin A, tetracycline, aspirin.
| [Side effects]
1. Changed skin and mucous membrane, including cheilitis, dry mouth, facial redness, dry eyes, conjunctivitis, etc;
2. Long-term use can cause liver and kidney damage;
3. Long-term application of Isotretinoin can cause osteoporosis, epiphyseal atresia, and retardation of bone formation. The incidence is <15%;
4. Teratogenic effect is one of the more serious adverse reactions.
| [Production methods]
β-ionone and ethyl chloride are first reacted together after which the product is further reacted with triphenylphosphine to obtain Triphenyl salt. The Triphenyl salt is further reacted with cyclopentenone derivative to produce isotretinoin and its 8Z isomer.
Separate out the 8Z isomer and convert it to isotretinoin through isomerization with the help of nitric acid.
|
|
|