| Identification | More | [Name]
Boc-O-tert-butyl-L-tyrosine | [CAS]
47375-34-8 | [Synonyms]
BOC-L-TYR(TBU)-OH
BOC-O-T-BUTYL-L-TYROSINE
BOC-O-TERT-BUTYL-L-TYROSINE
BOC-TYR(BUT)-OH
BOC-TYROSINE(TBU)-OH
BOC-TYR(TBU)-OH
N-ALPHA-T-BOC-O-T-BUTYL-L-TYROSINE
N-ALPHA-T-BUTOXYCARBONYL-O-T-BUTYL-L-TYROSINE
N-ALPHA-TERT-BUTYLOXYCARBONYL-O-TERT-BUTYL-L-TYROSINE
TIMTEC-BB SBB006926
N-tert-Butoxycarbonyl-O-tert-butyl-L-tyrosine
Boc-Tyr(tBul)-OH
N-ALPHA-T-BUTYLOXYCARBONYL-O-T-BUTYL-L-TYROSINE | [Molecular Formula]
C18H27NO5 | [MDL Number]
MFCD00065598 | [Molecular Weight]
337.41 | [MOL File]
47375-34-8.mol |
| Chemical Properties | Back Directory | [Melting point ]
113-118 °C
| [alpha ]
-15 º (C=1% IN DMF) | [Boiling point ]
484.0±40.0 °C(Predicted) | [density ]
1.116±0.06 g/cm3(Predicted) | [storage temp. ]
2-8°C
| [solubility ]
Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | [form ]
powder to crystal | [pka]
3.00±0.10(Predicted) | [color ]
White to Almost white | [optical activity]
[α]20/D 15.0±1.0°, c = 1% in DMF | [Water Solubility ]
Soluble in DMF. Insoluble in water. | [BRN ]
4327336 | [InChI]
InChI=1S/C18H27NO5/c1-17(2,3)23-13-9-7-12(8-10-13)11-14(15(20)21)19-16(22)24-18(4,5)6/h7-10,14H,11H2,1-6H3,(H,19,22)(H,20,21)/t14-/m0/s1 | [InChIKey]
ZEQLLMOXFVKKCN-AWEZNQCLSA-N | [SMILES]
C(O)(=O)[C@H](CC1=CC=C(OC(C)(C)C)C=C1)NC(OC(C)(C)C)=O | [CAS DataBase Reference]
47375-34-8(CAS DataBase Reference) |
| Hazard Information | Back Directory | [Chemical Properties]
White powder | [Uses]
N-Boc-O-tert-butyl-L-tyrosine is used as pharmaceutical intermediate. | [Application]
Boc-Tyr(tBu)-OH is an important organic reagent that can be used as a building block for the synthesis of many organic compounds. This compound could be used as a starting material to synthesize TPE-Tyr. TPE-Tyr can undergo covalent crosslinking via the formation of dityrosine linkages by exploiting horseradish peroxidase (HRP)-catalyzed phenol polymerization in the presence of H2O2, thereby activating the AIE process and switching on fluorescence emission of TPE core motif[1].
| [reaction suitability]
reaction type: Boc solid-phase peptide synthesis | [Synthesis]
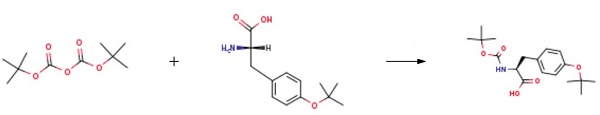
O-tert-butyl-L-tyrosine (2.00 g, 8.42 mmol, 1.0 equiv.) and di-tert-butyl dicarbonate (2.20 g, 10.10 mmol, 1.2 equiv.) were dissolved in a mixture of THF (15 mL) and NaOH (1 N, 30 mL) and stirred at room temperature for 10 h. THF was removed under reduced pressure, and the pH of residual solution was adjusted to 3.0 with dilute hydrochloric acid and the crude product was extracted by ethyl acetate. The combined organic phases was washed with saturated brine (pH 3.0) and dried over anhydrous Na2SO4. The organic solvent was removed under reduced pressure and dried in a vacuum oven at room temperature overnight, affording (N-Boc-O-tert-butyl)-L-Tyr (Boc-O-tert-butyl-L-tyrosine) as a yellowish solid (2.64 g, 93%)[1].
| [References]
[1] Wang X, et al. Highly Selective Fluorogenic Multianalyte Biosensors Constructed via Enzyme-Catalyzed Coupling and Aggregation-Induced Emission. Journal of the American Chemical Society, 2014; 136: 9890–9893.
|
|
|