| Identification | More | [Name]
Azathioprine | [CAS]
446-86-6 | [Synonyms]
6-[(1-methyl-4-nitro-1h-imidazol-5-yl)thio]-1h-purine
6-(1-METHYL-4-NITROIMIDAZOL-5-YL)THIOPURINE
6-(methyl-p-nitro-5-imidazolyl)-thiopurine
AZATHIOPRINE
AZATHIOPURINE
IMURAN
6-((1-methyl-4-nitro-1h-imidazol-5-yl)thio)-1h-purin
6-((1-methyl-4-nitroimidazol-5-yl)thio)-purin
6-(1’-methyl-4’-nitro-5’-imidazolyl)-mercaptopurine
6-(1-Methyl-4-nitro-5-imidazolythio)-9H-pur-ine
6-(1-methyl-4-nitroimidazol-5-ylthio)purin
6-(1-methyl-p-nitro-5-imidazolyl)-thiopurine
azamun
azanin
azathioprineusp
bw57-322
imurek
imurel
methylnitroimidazolylmercaptopurine
muran | [EINECS(EC#)]
207-175-4 | [Molecular Formula]
C5H5N5S | [MDL Number]
MFCD00069203 | [Molecular Weight]
167.19 | [MOL File]
446-86-6.mol |
| Chemical Properties | Back Directory | [Appearance]
Azathioprine is a complex heterocyclic
compound which forms pale yellow crystals. | [Melting point ]
243-244°C | [Boiling point ]
521.0±60.0 °C(Predicted) | [density ]
1.5379 (rough estimate) | [refractive index ]
1.7400 (estimate) | [storage temp. ]
Freezer | [solubility ]
Soluble in Dichloromethane and dimethyl sulfoxide. | [form ]
neat | [pka]
8.2(at 25℃) | [color ]
Pale-yellow crystals from Me2CO (aq) | [Stability:]
Stable. Incompatible with strong oxidizing agents, strong bases. | [biological source]
synthetic (organic) | [Water Solubility ]
<0.1 g/100 mL at 23 ºC | [Usage]
An immunosuppressive antimetabolite. Also active as disease modifying antirheumatic drug (DMARD) | [Merck ]
902 | [BCS Class]
4 | [Contact allergens]
This immunosuppressive and antineoplastic drug is
derived from 6-mercaptopurine. It caused allergic contact
dermatitis in a mother crushing tablets for her leukemic
son, and occupational dermatitis in a pharmaceutical
reconditioner of old tablet packaging machines, and in a
production mechanic working in packaging for a pharmaceutical
company. | [InChIKey]
LMEKQMALGUDUQG-UHFFFAOYSA-N | [CAS DataBase Reference]
446-86-6(CAS DataBase Reference) | [IARC]
1 (Vol. 26, Sup 7, 100A) 2012 | [EPA Substance Registry System]
446-86-6(EPA Substance) |
| Hazard Information | Back Directory | [Chemical Properties]
Yellow Solid | [Uses]
An immunosuppressive antimetabolite. Also active as disease modifying antirheumatic drug (DMARD). Azathioprine is a purine analog with immunosuppressive effects. | [Uses]
immunosuppressant, antineoplastic, antirheumatic | [Definition]
ChEBI: A thiopurine that is 6-mercaptopurine in which the mercapto hydrogen is replaced by a 1-methyl-4-nitroimidazol-5-yl group. It is a prodrug for mercaptopurine and is used as an immunosuppressant, prescribed for the treatment of inflammatory conditions and a
ter organ transplantation and also for treatment of Crohn's didease and MS. | [General Description]
Pale yellow crystals or yellowish powder. Decomposes at 243-244°C. Used for the treatment of rheumatoid arthritis. A known carcinogen. | [Reactivity Profile]
AZATHIOPRINE(446-86-6) may react exothermically with acids. Incompatible with isocyanates, peroxides, phenols, epoxides, anhydrides, and acid halides. Hydrolyzed by strongly basic solutions . May react with strong reducing agents to generate flammable gaseous hydrogen or hydrogen sulfide. | [Air & Water Reactions]
Sensitive to oxidation in the air. Insoluble in water. | [Hazard]
Confirmed carcinogen. | [Potential Exposure]
Azathioprine is an immunosuppressive
agent, generally used in combination with a corticosteroid
to prevent rejection following renal homotransplantations.
It also is used following transplantation of other organs.
Other uses of azathioprine include the treatment of a variety
of presumed autoimmune diseases, including rheumatoid
arthritis; ankylosing spondylitis; systemic lupus
erythematosus; dermatonyositis, periarteritis nodosa, scleroderma,
refractory thombocytopenic purpura; autoimmune
hemolytic anemia; chronic active liver disease; regional
enteritis; ulcerative colitis; various autoimmune diseases of
the eye; acute and chronic glomerulonephritis; the nephritic
syndrome; Wegener’s granulomatosis; and multiple
sclerosis. | [Fire Hazard]
Flash point data for this chemical are not available. AZATHIOPRINE is probably combustible. | [First aid]
Move victim to fresh air. Call 911 or emergency
medical service. Give artificial respiration if victim is not
breathing. Do not use mouth-to-mouth method if victim
ingested or inhaled the substance; give artificial respiration
with the aid of a pocket mask equipped with a one-way
valve or other proper respiratory medical device.
Administer oxygen if breathing is difficult. Remove and
isolate contaminated clothing and shoes. In case of contact
with substance, immediately flush skin or eyes with running
water for at least 20 minutes. For minor skin contact,
avoid spreading material on unaffected skin. Keep victim
warm and quiet. Effects of exposure (inhalation, ingestion
or skin contact) to substance may be delayed. Ensure that
medical personnel are aware of the material(s) involved
and take precautions to protect themselves. Medical observation
is recommended for 24 to 48 hours after breathing
overexposure, as pulmonary edema may be delayed. As
first aid for pulmonary edema, a doctor or authorized paramedic
may consider administering a drug or other inhalation
therapy. | [Shipping]
UN2811 Toxic solids, organic, n.o.s., Hazard
Class: 6.1; Labels: 6.1-Poisonous materials, Technical
Name Required. | [Incompatibilities]
Incompatible with reducing agents, such
as hydrides (may cause the release of explosive gases), oxidizers
(chlorates, nitrates, peroxides, permanganates, perchlorates,
chlorine, bromine, fluorine, etc.); contact may
cause fires or explosions. Keep away from alkaline materials,
strong acids (violent exothermic reaction), strong bases. | [Description]
This immunosuppressive and antineoplastic drug is
derived from 6-mercaptopurine. It caused occupational
dermatitis in a pharmaceutical worker, reconditioner
of old tablet packaging machines and in a production
mechanic, working in packaging for a pharmaceutical
company. | [Originator]
Imuran,Wellcome,UK,1964 | [Indications]
Azathioprine (Imuran) is a cytotoxic agent that preferentially
destroys any rapidly dividing cell. Since immunologically
competent cells are generally rapidly dividing
cells, azathioprine is very effective as an
immunosuppressive drug. Unfortunately, any cell that is
replicating is a target for this action. This lack of specificity
leads to serious side effects.
Azathioprine, in combination with corticosteroids,
has historically been used more widely than any other
drug in immunosuppressive therapy. It is classified as a
purine antimetabolite and is a derivative of 6-mercaptopurine. | [Manufacturing Process]
N,N'-Dimethyloxaldiamide is reacted with PCl5, to give 4-chloro-1-methyl
imidazole. This is nitrated with HNO3 to give 5-nitro-1-methyl-4-
chloroimidazole. Then, a mixture of 4.6 grams of anhydrous 6-
mercaptopurine, 5 grams of 1-methyl-4-chloro-5-nitroimidazole and 2.5 grams
of anhydrous sodium acetate in 100 ml of dry dimethyl sulfoxide was heated
at 100°C for 7 hours.
After standing overnight at room temperature, the mixture was poured into
200 ml of cold water and the yellow precipitate of 6-(1'-methyl-4'-nitro-5'-
imidazolyl)mercaptopurine (7.0 grams) collected. After recrystallization from
50% aqueous acetone, the product melted at 243-244°C, dec., and had an UV
spectrum with λ maximum = 280 nm at pH 1 and λ max. = 285 nm at pH 11. | [Brand name]
Imuran (Promethus). | [Therapeutic Function]
Immunosuppressive | [Biochem/physiol Actions]
Has shown promise in treatment of alopecia areata (autoimmune hair loss), with no difference in effectiveness between genders. | [Mechanism of action]
Azathioprine is a phase-specific drug that is toxic to
cells during nucleic acid synthesis. Phase-specific drugs
are toxic during a specific phase of the mitotic cycle,
usually the S-phase, when DNA synthesis is occurring,
as opposed to cycle-specific drugs that kill both cycling
and intermitotic cells.
Azathioprine is converted in vivo to thioinosinic
acid, which competitively inhibits the synthesis of inosinic
acid, the precursor to adenylic acid and guanylic
acid. In this way, azathioprine inhibits DNA synthesis
and therefore suppresses lymphocyte proliferation.This
effectively inhibits both humoral and cell-mediated immune
responses. | [Pharmacology]
Azathioprine is well absorbed following oral administration,
with peak blood levels occurring within 1 to 2
hours. It is rapidly and extensively metabolized to 6-
mercaptopurine, which is further converted in the liver
and erythrocytes to a variety of metabolites, including 6-
thiouric acid. Metabolites are excreted in the urine.The
half-life of azathioprine and its metabolites in the blood
is about 5 hours. | [Clinical Use]
Azathioprine is a relatively powerful antiinflammatory
agent. Although its beneficial effect in various conditions
is principally attributable to its direct immunosuppressive
action, the antiinflammatory properties of the
drug play an important role in its overall therapeutic effectiveness.
Azathioprine has been used widely in combination
with corticosteroids to inhibit rejection of organ transplants,
particularly kidney and liver allografts. However,
it is usually reserved for patients who do not respond to
cyclosporine plus corticosteroids alone.
Azathioprine also has applications in certain disorders
with autoimmune components, most commonly
rheumatoid arthritis. It is as effective as cyclophosphamide
in the treatment of Wegener’s granulomatosis.
It has largely been replaced by cyclosporine in immunosuppressive
therapy. Relative to other cytotoxic
agents, the better oral absorption of azathioprine is the
reason for its more widespread clinical use. | [Side effects]
The therapeutic use of azathioprine has been limited by
the number and severity of adverse effects associated
with its administration. Bone marrow suppression resulting
in leukopenia, thrombocytopenia, or both may
occur. GI toxicity may be a problem. It is also mildly hepatotoxic.
Because of its immunosuppressive activity,
azathioprine therapy can lead to serious infections. It
has been shown to be mutagenic in animals and humans
and carcinogenic in animals. | [Synthesis]
Azathioprine, 6-[(1-methyl-4-nitroimidazol-5-yl)thio]purine (31.2.1), is
synthesized by heteroarylation of the sulfhydrile group of 6-mercaptopurine (30.1.2.9) with
5-chloro-1-methyl-4-nitroimidazol in the presence of sodium acetate as a weak base.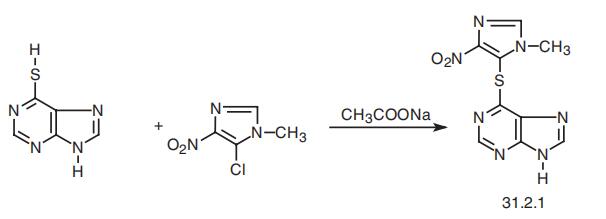
| [Veterinary Drugs and Treatments]
In veterinary medicine, azathioprine is used primarily as an immunosuppressive
agent in the treatment of immune-mediated
diseases in dogs. See Doses below for more information. For autoagglutinizing
immune mediated hemolytic anemia, azathioprine is
generally recommended to start at the time of diagnosis. When used
in combination with cyclosporine, azathioprine has been used to
prevent rejection of MHC-matched renal allografts in dogs.
Although the drug can be very toxic to bone marrow in cats, it is
sometimes used to treat feline autoimmune
skin diseases. | [Drug interactions]
Potentially hazardous interactions with other drugs
Allopurinol: enhances effect with increased
toxicity. Reduce azathioprine dose by 50-75% if
administered concomitantly - ideally avoid.
Antibacterials: increased risk of haematological
toxicity with co-trimoxazole.
Anticoagulants: possibly reduced anticoagulant effect
of coumarins.
Antipsychotics: avoid with clozapine.
Antivirals: myelosuppressive effects enhanced by
ribavirin.
Ciclosporin: decreased ciclosporin absorption and
bioavailability.
Cytotoxics may be additive or synergistic in
producing toxicity, particularly on the bone marrow.
Febuxostat: avoid concomitant use.
Vaccines: risk of generalised infections with live
vaccines - avoid. | [Carcinogenicity]
Azathioprine is known to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in humans. | [Metabolism]
Azathioprine is extensively metabolised to its active moiety
mercaptopurine, which in turn is activated intracellularly
by conversion to nucleotide derivatives. Mercaptopurine
is rapidly and extensively metabolised in the liver, by
methylation, oxidation and by the formation of inorganic
sulfates. Thiol methylation is catalysed by the enzyme
thiopurine methyltransferase (TPMT). TPMT activity is
highly variable in patients because of a genetic polymorphism
in the TPMT gene. About 10% of a dose of azathioprine is
reported to be split between the sulfur and the purine ring
to give 1-methyl-4-nitro-5-thioimidazole. The proportion of
different metabolites is reported to vary between patients.
Metabolites and small amounts of unchanged azathioprine
and mercaptopurine are eliminated in the urine. | [storage]
4°C, protect from light |
| Safety Data | Back Directory | [Hazard Codes ]
Xi,T,Xn | [Risk Statements ]
R45:May cause cancer.
R22:Harmful if swallowed.
R36/37/38:Irritating to eyes, respiratory system and skin .
R20/21/22:Harmful by inhalation, in contact with skin and if swallowed . | [Safety Statements ]
S53:Avoid exposure-obtain special instruction before use .
S22:Do not breathe dust .
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36/37:Wear suitable protective clothing and gloves .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) .
S36:Wear suitable protective clothing . | [WGK Germany ]
3 | [RTECS ]
UO8925000 | [Hazard Note ]
Irritant | [HazardClass ]
IRRITANT | [HS Code ]
29339900 | [Safety Profile]
Confirmed human
carcinogen producing bladder tumors and
leukemia. Poison by subcutaneous,
intradermal, and intraperitoneal routes.
Moderately toxic by ingestion. Human
systemic effects: liver changes,
hypermotility, diarrhea, nausea or vomiting,
increased body temperature, BP lowering,
decreased urine volume or anuria,
normocytic anemia, bone marrow changes.
An experimental teratogen. Other
experimental reproductive effects. Human
mutation data reported. When heated to
decomposition it emits very toxic fumes of
NO,xand SOx. An immunosuppressant. | [Hazardous Substances Data]
446-86-6(Hazardous Substances Data) | [Toxicity]
LD50 orl-rat: 535 mg/kg NIIRDN 6,3,82 |
| Questions And Answer | Back Directory | [Application in Particular Diseases]
In Rheumatic Arthritis:
Azathioprine is a purine analog that is converted to 6-mercaptopurine and is thought to interfere with DNA and RNA synthesis. Antirheumatic effects may be seen in 3 to 4 weeks. It should be discontinued if no response is observed after 12 weeks at maximal doses. Its major adverse effects are bone marrow suppression (leukopenia, macrocytic anemia, thrombocytopenia, pancytopenia), stomatitis, GI intolerance, infections, drug fever, hepatotoxicity, and oncogenic potential.
|
|
|