| Identification | More | [Name]
7-Dehydrocholesterol | [CAS]
434-16-2 | [Synonyms]
3BETA-HYDROXY-5,7-CHOLESTADIENE
5,7-CHOLESTADIEN-3BETA-OL
5,7-CHOLESTADIEN-3-BETA-OL MONOHYDRATE
(-)-7-DEHYDROCHOLESTEROL
7-DEHYDROCHOLESTEROL
7-DEHYDROCHOLESTEROL MONOHYDRATE
DEHYDROCHOLESTEROL, 7-
PROVITAMIN D3
PROVITAMIN D3 MONOHYDRATE
(3)-7-Dehydrochlesterol
(3beta)-Cholesta-5,7-dien-3-ol
(3-beta)-cholesta-7-dien-3-ol
(3beta)-cholesta-7-dien-3-ol
3-Hydroxycholesta-5,7-diene
5,7-Cholestandien-3.beta.-ol
5,7-Cholestandien-3beta-ol
7,8-Didehydrocholesterol
7-Dehydrocholesterin
7-dehydro-cholestero
7-dien-3-ol,(3.beta.)-Cholesta-5 | [EINECS(EC#)]
207-100-5 | [Molecular Formula]
C27H44O | [MDL Number]
MFCD00003624 | [Molecular Weight]
384.64 | [MOL File]
434-16-2.mol |
| Chemical Properties | Back Directory | [Appearance]
white to off-white fine crystalline powder | [Melting point ]
148-152 °C(lit.)
| [alpha ]
-116 º (C=1, CHCL3) | [Boiling point ]
451.27°C (rough estimate) | [density ]
0.9717 (rough estimate) | [refractive index ]
1.5100 (estimate) | [storage temp. ]
−20°C
| [solubility ]
Chloroform (Sparingly), DMSO (Slightly, Heated, Sonicated), Ethyl Acetate (Slightly) | [form ]
Solid | [pka]
14.91±0.70(Predicted) | [color ]
White to Yellow | [biological source]
synthetic | [optical activity]
[α]20/D -115±8°, c = 1% in chloroform | [Water Solubility ]
Insoluble | [Merck ]
13,2887 | [BRN ]
2224615 | [Stability:]
Light Sensitive | [InChIKey]
UCTLRSWJYQTBFZ-DDPQNLDTSA-N | [LogP]
9.460 (est) | [CAS DataBase Reference]
434-16-2(CAS DataBase Reference) | [NIST Chemistry Reference]
Cholesta-5,7-dien-3-ol, (3«beta»)-(434-16-2) | [EPA Substance Registry System]
434-16-2(EPA Substance) |
| Questions And Answer | Back Directory | [Vitamin Precursor]
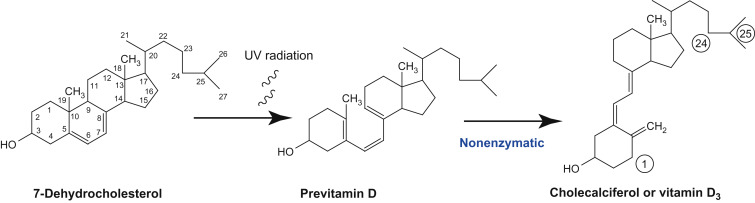
7-Dehydrocholesterol is a natural active ingredient found in the skin. In the epithelial cells of the intestinal wall, cholesterol is oxidised to 7-Dehydrocholesterol, which is then transported to the skin. Exposure to UV radiation converts 7-Dehydrocholesterol into an activated form of the vitamin precursor (cholecalciferol). The reaction mechanism is that the conjugated diene group of 7-Dehydrocholesterol absorbs UV radiation in the wavelength range of 250-310 nm, leading to the opening of the B-ring of the steroid nucleus and the formation of pro-vitamin D, which is then isomerised in a non-enzymatic reaction to form cholecalciferol, also known as vitamin D3.
 |
| Hazard Information | Back Directory | [Chemical Properties]
white to off-white fine crystalline powder. It is easy to oxidize when exposed to air. | [Uses]
7-Dehydrocholesterol(434-16-2) is a vitamin D3 precursor, which is a sterol found in animals. Vitamin D3 is produced by the action of sunlight or ultraviolet irradiation from the precursor, 7-dehydrocholesterol (7-DC), that is synthesized in the skin of animals and humans.
In most tissues of the body, 7-dehydrocholesterol is the immediate precursor of cholesterol. It is reduced to cholesterol by the enzyme 3β-hydroxysterol-Δ7-reductase (DHCR7) in the last step of cholesterol biosynthesis. 7-DHC accumulates in Smith-Lemli-Opitz syndrome (SLOS), a disorder characterized by a mutation in the DHCR7 gene and decreased cholesterol levels in bodily tissues and fluids, as well as microcephaly, intellectual disability, and distinctive dysmorphic features. It is highly susceptible to free radical oxidation, giving rise to several oxysterols that may be involved in the pathogenesis of SLOS. 7-DHC levels are increased in brain, liver, and serum in a rat model of SLOS induced by the DHCR7 inhibitor AY 9944. 7-DHC is a provitamin that is converted to vitamin D3 by ultraviolet-B (UVB) light in a human skin equivalent system and in isolated human skin samples. | [Definition]
ChEBI: 7-Dehydrocholesterol is a 3β-sterol, a cholestanoid, a Delta(5),Delta(7)-sterol and a 3beta-hydroxy-Delta(5)-steroid. It has a role as a human metabolite and a mouse metabolite. | [General Description]
7-Dehydrocholesterol, a 5,7-conjugated diene sterol is a biosynthetic precursor of cholesterol. It helps in the production of vitamin D3 when exposed to ultraviolet B (UVB) radiation. It has been used as an internal standard to determine sterols. 7-dehydrocholesterol is also used in the diagnosis of Smith-Lemli-Opitz syndrome. Blood and tissue of infants with Smith- Lemli-Opitz (SLO) syndrome contain reduced amounts of cholesterol and greatly increased concentrations of 7-dehydrocholesterol and of its isomer 8-dehydrocholesterol. | [Biochem/physiol Actions]
Down-regulates cholesterol biosynthesis in cultured Smith-Lemi-Opitz syndrome skin fibroblasts. |
|
|