| Identification | More | [Name]
1-Prop-2-enoxy-4-(4-prop-2-enoxyphenyl)sulfonyl-benzene | [CAS]
41481-63-4 | [Synonyms]
1,1'-sulfonylbis[4-(2-propenyl)oxy-benzene]
1-prop-2-enoxy-4-(4-prop-2-enoxyphenyl)sulfonyl-benzene
BIS(4-ALLYLOXYPHENYL)SULFONE
1,1'-PHENYLSULFONYL-4,4'-DIENEPROPYL ETHER
1,1'-Phenylsulfonyl-4,4'-Dienepropyl(2)Ether
Bis-[4-(2propenyloxy)-phenyl]-sulfone
Bis(p-allyloxyphenyl) sulfone
Sulfonylbis(4,1-phenylene)bis(allyl ether) | [EINECS(EC#)]
1533716-785-6 | [Molecular Formula]
C18H18O4S | [MDL Number]
MFCD00506306 | [Molecular Weight]
330.4 | [MOL File]
41481-63-4.mol |
| Chemical Properties | Back Directory | [Melting point ]
141-142 °C(Solv: ethanol (64-17-5)) | [Boiling point ]
500.7±45.0 °C(Predicted) | [density ]
1.171±0.06 g/cm3(Predicted) | [storage temp. ]
-20°C Freezer, Under inert atmosphere | [solubility ]
DMSO (Slightly), Methanol (Slightly) | [form ]
Solid | [color ]
White to Off-White | [CAS DataBase Reference]
41481-63-4(CAS DataBase Reference) |
| Hazard Information | Back Directory | [Chemical Properties]
White crystalline powder | [Preparation]
The preparation of 1,1'-Sulfonylbis[4-(prop-2-en-1-yloxy)benzene is as follows:2400g water, 216g caustic soda lye of 48% strength, 600g 4,4'-dihydroxydiphenyl sulfone and 204g Allyl chloride were mixed and the reaction was carried out at 55-60°C and autogenously generated pressure of 0.8-1. Okg/cm2 for 8 hours. 204g caustic soda lye of 48% strength was added in the reaction mixture, and maintained for 1 hour at 80°C thereafter filtered at 80°C to remove 4,4'-diallyloxydiphenyl sulfone. Further, the filtrate solution was treated with 5g activated carbon and 5g sequestering agent at 85- 90°C. The pH of filtrated solution was adjusted to 8.9 with 20% sulfuric acid and cooled to 35°C. The precipitate was separated by filtration and slurred in water. The pH was adjusted to 4.94 with 20% sulfuric acid and the precipitate was separated by filtration, and washed with water and dried to obtain 615g the product 4-allyloxy 4'-hydroxydiphenyl sulfone of 96% purity. The yield was 93.5% with respect to 4,4'-dihydroxydiphenyl sulfone. The wet cake of 4,4'-diallyloxydiphenyl sulfone was slurred in 100g of 5%w/v aqueous solution of sodium hydroxide and refluxed for 1 hour, then cooled to 80°C. The precipitate was filtered, washed with water and dried to obtain 45g of 4,4'-diallyloxy diphenyl sulfone of 91.9% purity. The yield was 6% with respect to 4,4'-dihydroxydiphenyl sulfone. pH of the mother liquor obtained after separation of 4-allyloxy 4'-hydroxydiphenyl sulfone, was adjusted to 4.5 with 20% sulfuric acid. The precipitate was separated by filtration, washed with water and dried to obtain 33g of 4,4'-dihydroxydiphenyl sulfone of 87.9% purity. The conversion of 4,4'-dihydroxydiphenyl sulfone was 94.50%.; 595g of 4-allyloxy 4'-hydroxydiphenyl sulfone of 96% purity was dissolved in 230g of 48% strength caustic soda lye and 1000g water, treated with 5g activated carbon and 5g sequestering agent at 85-90°C. The slurry was filtered and pH of the filtrate was adjusted to 8.95 with 20% sulfuric acid and then cooled to 35°C. The precipitate was separated by filtration, slurried in water and the pH was adjusted to 5.0 with 20% sulfuric acid. The slurry was filtrated and the precipitate was washed with water and dried to obtain 563g of 4- allyloxy 4'-hydroxydiphenyl sulfone of 99.273% purity.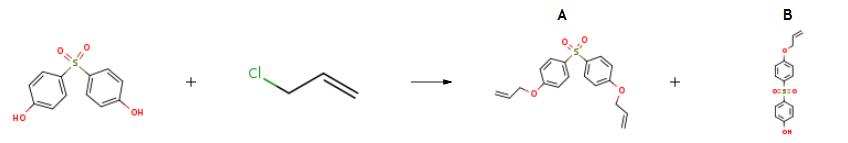
|
|
|