| Identification | More | [Name]
COPPER(II) TRIFLUOROMETHANESULFONATE | [CAS]
34946-82-2 | [Synonyms]
COPPER(II) TRIFLATE
COPPER(II) TRIFLUOROMETHANESULFONATE
COPPER(II) TRIFLUOROMETHANESULPHONATE
COPPER TRIFLATE
CUPRIC TRIFLUOROMETHANESULFONATE
TRIFLUOROMETHANESULFONIC ACID COPPER(II) SALT
TRIFLUOROMETHANESULFONIC ACID COPPER SALT
Copper (II) trifluoromethanesulfonate, (Copper triflate)
COPPER(II) TRIFLUOROMETHANESULFONATE, 98 %
Copper(II)trifluoromethanesulphonate,98%
Copper(II)trifluoromethanesulfonate,98%(Coppertriflate)
Copper(II) trifluoromethanesulphonate 98%
COPPER TRIFLUOROMETHANESULFONATE
coppertrifluoromethanesulfonate
Trifluoromethanesulfonic acid cupric salt
CUPRIC-2-TRIFLATE
Copper(II) trifluoromethanesulfonate, 99% min
Copper(II) triflate, Cupric trifluoromethanesulfonate, Trifluoromethanesulfonic acid copper(II) salt
Bis(trifluoromethanesulfonic acid)copper(II) salt
Bis(trifluoromethylsulfonyloxy) copper(II) | [EINECS(EC#)]
252-300-8 | [Molecular Formula]
C2CuF6O6S2 | [MDL Number]
MFCD00077492 | [Molecular Weight]
361.68 | [MOL File]
34946-82-2.mol |
| Chemical Properties | Back Directory | [Appearance]
white to slightly blue or light grey cryst. powder | [Melting point ]
≥300 °C
| [storage temp. ]
Inert atmosphere,Room Temperature | [solubility ]
Water (Slightly) | [form ]
Powder | [color ]
White to slightly blue or light gray | [Water Solubility ]
Soluble in water. | [Hydrolytic Sensitivity]
6: forms irreversible hydrate | [Sensitive ]
Hygroscopic | [BRN ]
4028198 | [Exposure limits]
ACGIH: TWA 1 mg/m3
NIOSH: IDLH 100 mg/m3; TWA 1 mg/m3 | [Stability:]
hygroscopic | [InChIKey]
SBTSVTLGWRLWOD-UHFFFAOYSA-L | [CAS DataBase Reference]
34946-82-2(CAS DataBase Reference) |
| Safety Data | Back Directory | [Hazard Codes ]
C,Xi | [Risk Statements ]
R34:Causes burns. | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) .
S27:Take off immediately all contaminated clothing . | [RIDADR ]
UN 3261 8/PG 2
| [WGK Germany ]
3
| [F ]
3-10 | [Hazard Note ]
Irritant/Hygroscopic | [TSCA ]
No | [HazardClass ]
8 | [PackingGroup ]
III | [HS Code ]
29049090 |
| Hazard Information | Back Directory | [Chemical Properties]
white to slightly blue or light grey cryst. powder | [Uses]
Copper(II) trifluoromethanesulfonate is a mild lewis acid. It is used as catalyst which promotes dehydration of alcohols and diols to alkenes at ambient temperatures. It is widely used to generate carbenoid species from ?-diazo esters and ketones, via in situ reduction to the Cu(I) species. It is also promotes the reaction between diazo esters and imines to give aziridines. It catalyzes syn-selective aldol condensation of (Z)-silyl enol ethers with aldehydes, Friedel-Crafts alkylation, acylation reactions of aromatics and addition of trimethylsilyl cyanide to carbonyl compounds. | [Preparation]
Copper(II) trifluoromethanesulfonate is prepared from copper(II) carbonate and triflic acid (Trifluoromethanesulfonic Acid) in MeCN.
| [reaction suitability]
core: copper
reagent type: catalyst | [Purification Methods]
Dissolve it in MeCN, add dry Et2O until cloudy and cool at -20o in a freezer. The light blue precipitate is collected and dried in a vacuum oven at 130o/20mm for 8hours. It has 737nm (� 22.4 max M1cm -1) in AcOH. [Salomon & Kochi J Am Chem Soc 95 330 1973]. It has also been dried in a vessel at 0.1Torr by heating with a Fischer burner [Andrist et al. J Org Chem 43 3422 1978]. It has been dried at 110-120o/5mm for 1hour before use and forms a *benzene complex which should be handled in a dry box because it is air sensitive [Kobayashi et al. Chem Pharm Bull Jpn 28 262 1980, Salomon & Kochi J Am Chem Soc 95 330 1973]. [Beilstein 3 IV 34.] |
| Questions And Answer | Back Directory | [Reaction]
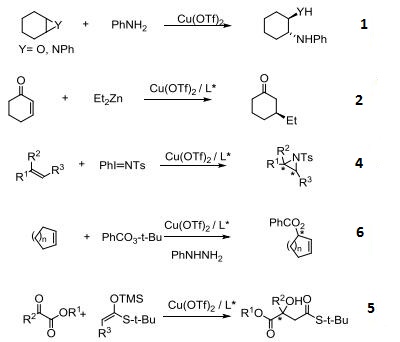
- Ring-Opening of epoxides and aziridines.
- Asymmetric conjugate addition of organozinc reagents to α,β-unsaturated ketones.
- Electrophilic addition of olefins.
- Asymmetric aziridination of olefins.
- Asymmetric cycloadditions and aldol condensations.
- Asymmetric Kharasch oxidation.
- Asymmetric Michael addition of enamides.
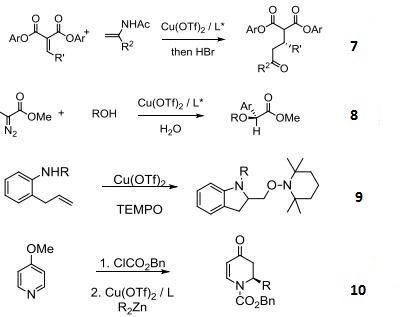
- Asymmetric O-H or O-R insertion reactions.
- Enantioselective intramolecular aminooxygenation of alkenes.
- Enantioselective addition of dialkylzinc reagents to N-acylpyridinium salts.
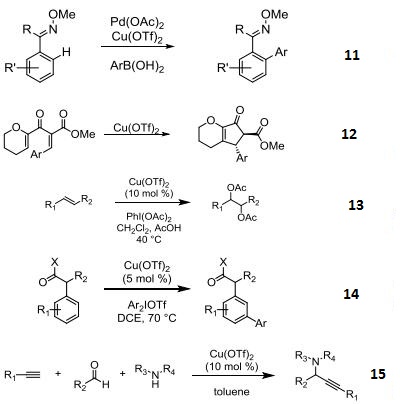
- Pd-catalyzed C-H functionalizations of oximes with arylboronic acids.
- Used as a Lewis acid in the Nazarov cyclization.
- Catalyst in the diacetoxylation olefins.
- Catalyst in the meta-selective direct arylation of α-aryl carbonyl compounds.
- Catalyst in the three-component coupling of amines, aldehydes, and alkynes.
|
|
|