| Identification | More | [Name]
3-(3-CHLORO-PHENYL)-3-OXO-PROPIONIC ACID ETHYL ESTER | [CAS]
33167-21-4 | [Synonyms]
3-(3-CHLORO-PHENYL)-3-OXO-PROPIONIC ACID ETHYL ESTER
ETHYL 3-(3-CHLOROPHENYL)-3-OXOPROPANOATE
ETHYL (3-CHLOROBENZOYL)ACETATE | [Molecular Formula]
C11H11ClO3 | [MDL Number]
MFCD03424810 | [Molecular Weight]
226.66 | [MOL File]
33167-21-4.mol |
| Hazard Information | Back Directory | [Uses]
Reagent / reactant involved in:• ;Oxidative cross-coupling via dioxygen activation with indoles1• ;Chlorination and hydrosilylation for synthesis of α-hydroxy β-amino acid derivatives2• ;Precursor for substrates for chiral Lewis base-catalyzed stereoselective reduction with trichlorosilane and water3• ;Intramolecular cyclization for synthesis of dihydrofurans4• ;Rate acceleration of Michael reactions5• ;Cerium ammonium nitrate-mediated oxidative coupling6 | [Synthesis]
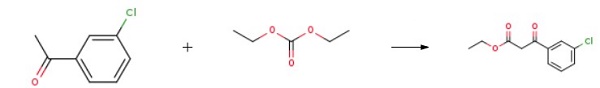
141.6 g of diethyl carbonate was dissolved in 1000 mL of a 1:1 ethanol-tetrahydrofuran (THF) solution, and 12.4 g of catalyst 1 Amberlite IRA-400 was added while stirring. The mixture was then heated up to 45 °C. In a separate container, 154.5 g of m-chloroacetophenone was dissolved in 1000 mL of the above-mentioned ethanol-THF mixed solvent. The addition of m-chloroacetophenone was completed within 1 hour, and stirring was continued for 6 hours. The mixture was then filtered. The filtrate was combined and the majority of the solvent was evaporated. 1500 mL of water was added to the remaining solution while stirring. The solution was then subjected to CH2Cl2 extraction, followed by washing with saturated saline until the solution reached a neutral pH. The resulting solution was dried with anhydrous MgSO4, filtered, and the solvent was evaporated. Finally, the resulting residue was distilled under reduced pressure to obtain a colorless oily liquid known as Ethyl (3-chlorobenzoyl)acetate.
 |
|
|