| Identification | More | [Name]
DIMETHYLKETENE METHYL TRIMETHYLSILYL ACETAL | [CAS]
31469-15-5 | [Synonyms]
1-METHOXY-1-(TRIMETHYLSILOXY)-2-METHYL-1-PROPENE
1-METHOXY-1-TRIMETHYLSILOXY-2-METHYLPROPENE
[(1-METHOXY-2-METHYL-1-PROPENYL)OXY]TRIMETHYLSILANE
1-METHOXY-2-METHYL-1-(TRIMETHYLSILOXY)PROPENE
DIMETHYLKETENE METHYL TRIMETHYLSILYL ACETAL
METHYL TRIMETHYLSILYL DIMETHYLKETENE ACETAL
MTDA
1-Methoxy-1-trimethylsiloxy-2-methyl-1-propene~Methyl trimethylsilyl dimethylketene acetal~MTDA
1-METHOXY-2-METHYL-1-TRIMETHYL-
Silane, ((1-methoxy-2-methyl-1-propenyl)oxy)trimethyl-
Methyl Trimethylsiliyl dimethyl ketene acetal
1-methoxy-2-methyl-1-trimethylsiloxypropane
(1-Methoxy-2-methyl-1-propenyloxy)trimethylsilane, MTDA
(1-Methoxy-2-methyl-1-propenyloxy)trimethylsilane, 1-Methoxy-2-methyl-1-(trimethylsiloxy)propene, MTDA
(1-Methoxy-2-methyl-1-propenyl)(trimethylsilyl) ether
(Trimethylsilyl)(1-methoxy-2-methyl-1-propenyl) ether
1-[(Trimethylsilyl)oxy]-1-methoxy-2-methyl-1-propene
2-Methyl-1-(trimethylsiloxy)-1-methoxy-1-propene | [EINECS(EC#)]
629-515-4 | [Molecular Formula]
C8H18O2Si | [MDL Number]
MFCD00010232 | [Molecular Weight]
174.31 | [MOL File]
31469-15-5.mol |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R10:Flammable.
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S16:Keep away from sources of ignition-No smoking .
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36:Wear suitable protective clothing . | [RIDADR ]
UN 3271 3/PG 3
| [WGK Germany ]
3
| [F ]
10-21 | [TSCA ]
Yes | [HazardClass ]
3 | [PackingGroup ]
III | [HS Code ]
29319090 |
| Hazard Information | Back Directory | [Chemical Properties]
Clear colorless liquid | [Physical properties]
bp 35 °C/15 mmHg; d 0.858 g cm?3. | [Uses]
1-Methoxy-2-methyl-1-(trimethylsilyloxy)
propene is widely used as functional equivalent of enolate of methyl isobutyrate; ester enolate
surrogate in electrophilic reactions including alkylation, aldol
reaction, Michael reaction, initiator for group transfer
polymerization of acrylates, nitroarylation, oxidation,
dimerization, and cycloadditions. | [Preparation]
The title compound is a prototypical ketene silyl acetal
(KSA) that can been prepared by either of the two most commonly
employed methods: (a) deprotonation of the |á-hydrogen of
an ester followed by silylation (1),16 and (b) metal-catalyzed
hydrosilylation of |á,|?-unsaturated esters(2).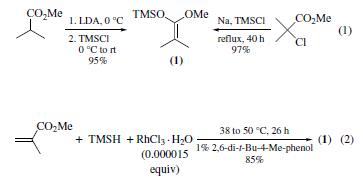
| [Purification Methods]
Add Et2O, wash with cold H2O, dry (Na2SO4), filter, evaporate Et2O, and distil the oily residue in a vacuum. [Ainsworth et al. J Organometal Chem 46 59 1972.] |
|
|