| Identification | More | [Name]
tert-Butyl peroxy-2-ethylhexanoate | [CAS]
3006-82-4 | [Synonyms]
2-ethyl-hexaneperoxoic acid 1,1-dimethylethyl ester
TERT-BUTYL PEROXY-2-ETHYLHEXANOATE
Tert-Butylperoxy-2-Ethylhecanoate
tert-butyl 2-ethylperoxyhexanoate
TERTIARY BUTYL PEROXY 2-ETHYL CAPROIC ACID ESTER
tert-Butyl peroctoate
tert-Butyl peroxyoctoate
Hexaneperoxoic acid, 2-ethyl-, 1,1-dimethylethyl ester
tert.-Butyl-2-ethylperoxyhexanoat
butyl peroxy-2-ethylhexanoate
Initiating agent OT
tert-butyl peroxy-2-ethyl-hexanoate,[with 2,2di-(tert-butyl peroxy)-butane]
T-BUTYLPEROXY-2-ETHYLHEXANOATE
tert-Butyl 2-ethylhexaneperoxoate
tert-Butyl peroxy-2-ethylhexanoate, not more than 50%, with phlegmatizer (UN 2888)
(2-Ethylhexanoyl)(tert-butyl) peroxide
2-Ethylhexaneperoxoic acid 1,1-dimethylethyl
2-Ethylhexaneperoxoic acid tert-butyl ester
Trigonox 21
t-butylperoxyoctoate | [EINECS(EC#)]
221-110-7 | [Molecular Formula]
C12H22O4 | [MDL Number]
MFCD00072690 | [Molecular Weight]
230.3 | [MOL File]
3006-82-4.mol |
| Chemical Properties | Back Directory | [Melting point ]
-30°C | [Boiling point ]
248.9±23.0 °C(Predicted) | [density ]
0.89 | [vapor pressure ]
2Pa at 20℃ | [Fp ]
85°C | [pka]
-4.8[at 20 ℃] | [Water Solubility ]
46.3mg/L at 20℃ | [InChI]
InChI=1S/C12H24O3/c1-6-8-9-10(7-2)11(13)14-15-12(3,4)5/h10H,6-9H2,1-5H3 | [InChIKey]
WYKYCHHWIJXDAO-UHFFFAOYSA-N | [SMILES]
C(OOC(C)(C)C)(=O)C(CC)CCCC | [LogP]
4.79 at 20℃ | [CAS DataBase Reference]
3006-82-4(CAS DataBase Reference) | [EPA Substance Registry System]
3006-82-4(EPA Substance) |
| Hazard Information | Back Directory | [General Description]
This peroxide is particularly sensitive to temperature rises and contamination. Above a given "Control Temperature" they decompose violently. TERT-BUTYL PEROXY-2-ETHYLHEXANOATE, NOT MORE THAN 50%, WITH PHLEGMATIZER(3006-82-4) is generally stored or transported with inert solids to mitigate the explosion hazard. | [Reactivity Profile]
TERT-BUTYL PEROXY-2-ETHYL-HEXANOATE explodes with great violence when rapidly heated to a critical temperature; pure form is shock sensitive and detonable [Bretherick 1979 p. 602]. Decomposes violently or explosively at temperatures 0-10°C owing to self-accelerating exothermic decomposition; Several explosions were due to shock, heat or friction; amines and certain metals can cause accelerated decomposition [Bretherick 1979 p. 156]. Danger of explosion when dry | [Chemical Properties]
t-Butyl peroxy-2-ethylhexanoate is a colorless liquid. It has a
half-life in benzene of 10.0 h at 162°F (72°C). It is used as a
medium temperature initiator for the polymerization of vinyl
monomers and the curing of styrene-unsaturated polyester
resins. It is regarded as an intermediate fire hazard; however,
it has low impact sensitivity . | [Uses]
Tert-Butyl peroxy-2-ethylhexanoate (TBPO), also known as Trigonox 21S or PEROXAN PO-30 , is an initiator for (co)polymerization of ethylene, styrene, acrylonitrile and (meth)acrylates. Curing agent for unsaturated polyester resins. It is used for high pressure polymerization of ethylene in both autoclave and tubular processes, usually in combination with other peroxides of varying degrees of activity. | [Flammability and Explosibility]
Notclassified | [Safety Profile]
Based on available data��,tert-Butyl peroxy-2-ethylhexanoate is considered to be very toxictowards aquatic organisms and is classified according to EC 1272/2008 regulation.
Several tests have shown that tert-Butyl peroxy-2-ethylhexanoate is degradable by hydrolysisand also by biotic degradation in water: this substance is inherently biodegradable.As aconsequence, tert-Butyl peroxy-2-ethylhexanoate is neither PBT nor vPvB. | [Synthesis]
The synthesis method of commercial peroxyester tert-butyl peroxy-2-ethylhexanoate (TBPEH) is shown below:
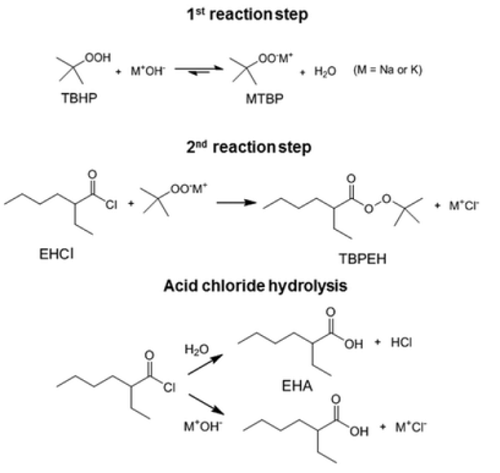
The first reaction step is the deprotonation of tert-butyl hydroperoxide (TBHP) with the base (MOH) in the aqueous phase, a homogeneous and slightly exothermic reaction. The base is usually in excess with respect to TBHP to ensure a high enough pH when the reaction has almost completed. Thanks to the TBHP acidity, the equilibrium is shifted to the right and most of the TBHP present in the aqueous phase is deprotonated. The alkali salt of TBHP (MTBP) further reacts with 2-ethylhexanoyl chloride (EHCl) to form TBPEH and a chloride salt (MCl). The reaction is biphasic with MTBP, MOH, MCl and some TBHP in the aqueous phase and EHCl, TBPEH and most of TBHP in the organic phase. The reaction between EHCl and TBHP is very slow and can be neglected[1].
| [Toxics Screening Level]
The ITSL for t-butylperoxy-2-ethylhexanoate has been changed from 0.04 μg/m3 to
0.1 μg/m3 based on an annual averaging time. | [References]
[1] Magosso, M. M. van den Berg and J. van der Schaaf. “Kinetic study and modeling of the Schotten–Baumann synthesis of peroxyesters using phase-transfer catalysts in a capillary microreactor.” Reaction Chemistry & Engineering 9 (2021): 1574–1590.
|
|
|