| Identification | More | [Name]
4-Nitrophenethylamine hydrochloride | [CAS]
29968-78-3 | [Synonyms]
2-(4-NITROPHENYL)ETHYLAMINE HYDROCHLORIDE
2-(P-NITROPHENYL)ETHYLAMINE HYDROCHLORIDE
4-NITROPHENETHYLAMINE HCL
4-NITROPHENETHYLAMINE HYDROCHLORIDE
4-NITROPHENYLETHYLAMINE HCL
4-NITROPHENYLETHYLAMINE HYDROCHLORIDE
p-Nitrophenethylamine hydrochloride
4-Nitrophcnethylamine
4-NITROPHENYLETHYLAMINO HYDROCHLORIDE
4-NITROPHENYLAMINEHCL
2-(4-NITROPHENYL)ETHYLAMINE HYDROCHLORIDE 97+%
P-NITROPHENYLETHYLAMINE HCL
2-(4-nitrophenyl) ethylamine, HCl
Benzeneethanamine, 4-nitro-, monohydrochloride | [EINECS(EC#)]
249-980-3 | [Molecular Formula]
C8H11ClN2O2 | [MDL Number]
MFCD00012900 | [Molecular Weight]
202.64 | [MOL File]
29968-78-3.mol |
| Chemical Properties | Back Directory | [Appearance]
yellow to brown crystalline powder and chunks | [Melting point ]
200 °C (dec.)(lit.)
| [storage temp. ]
0-6°C | [solubility ]
Soluble in methanol almost transparency. | [form ]
Crystalline Powder | [color ]
Yellow to yellow-green | [BRN ]
3568915 | [Stability:]
Hygroscopic | [InChIKey]
JVMHULJEYUQYSH-UHFFFAOYSA-N | [CAS DataBase Reference]
29968-78-3(CAS DataBase Reference) |
| Safety Data | Back Directory | [Hazard Codes ]
Xn | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin .
R40:Limited evidence of a carcinogenic effect. | [Safety Statements ]
S22:Do not breathe dust .
S24/25:Avoid contact with skin and eyes .
S36:Wear suitable protective clothing .
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice . | [WGK Germany ]
3
| [HS Code ]
29214990 |
| Hazard Information | Back Directory | [Chemical Properties]
yellow to brown crystalline powder and chunks | [Uses]
4-Nitrophenethylamine hydrochloride was used in the synthesis of ortho-metalated primary phenethylamines complexes containing six-membered palladacycles and N-(4-nitrophenethyl)formamide. | [Preparation]
The preparation of 4-Nitrophenethylamine hydrochloride is as follows:Ten mL of 2M HCl was then added in a one-pot fashion, and, in all cases but threonine, the reaction vessel was heated to 190°Cover 5 min with stirring and then allowed to cool. In the case of the temperature-sensitive threonine, soxhlet extraction with toluene was performed at 80°C to remove R-carvone from the reaction mixture. The aqueous reaction mixture was washed three times with 25 mL of ether, and then water solvent was distilled off from the hydrochloride salt. The hydrochloride salt was transferred to a vacuum oven and dried overnight at 150 °C and 10 Torr.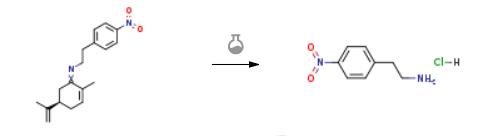
|
|
|