| Identification | More | [Name]
2-(Dicyclohexylphosphino)biphenyl | [CAS]
247940-06-3 | [Synonyms]
(2-BIPHENYLYL)DICYCLOHEXYLPHOSPHINE
2-(DICYCLOHEXYLPHOSPHINO)BIPHENYL
2-(DICYLOHEXYLPHOSPHINO)BIPHENYL
CYCLOHEXYL JOHNPHOS
2-(Dicyclohexylphosphino)biphenyl2-(Dicyclohexylphosphino)biphenyl
2-(Dicyclohexylphosphino)biphenyl,98%
(2-biphenyl)dicyclohexylphosphine
2-(DICYCLOHEXYLPHOSPHINO)BIPHENYL 98%
PHOSPHINE, [1,1''-BIPHENYL]-2-YLDICYCLOHEXYL-
2-(Dicyclohexylphosphino)biphenyl, Cyclohexyl JohnPhos | [EINECS(EC#)]
480-030-2 | [Molecular Formula]
C24H31P | [MDL Number]
MFCD01862441 | [Molecular Weight]
350.48 | [MOL File]
247940-06-3.mol |
| Chemical Properties | Back Directory | [Appearance]
white to light yellow crystal powde | [Melting point ]
102-106 °C(lit.)
| [Boiling point ]
499.5±24.0 °C(Predicted) | [storage temp. ]
Inert atmosphere,2-8°C | [solubility ]
soluble in Toluene | [form ]
crystal | [color ]
white | [Sensitive ]
Air Sensitive | [Usage]
Ligand employed in an extremely general method for the Pd-catalyzed synthesis of aromaticamines using aryl chlorides, bromides and triflates. | [BRN ]
8440533 | [InChI]
InChI=1S/C24H31P/c1-4-12-20(13-5-1)23-18-10-11-19-24(23)25(21-14-6-2-7-15-21)22-16-8-3-9-17-22/h1,4-5,10-13,18-19,21-22H,2-3,6-9,14-17H2 | [InChIKey]
LCSNDSFWVKMJCT-UHFFFAOYSA-N | [SMILES]
P(C1=CC=CC=C1C1=CC=CC=C1)(C1CCCCC1)C1CCCCC1 | [CAS DataBase Reference]
247940-06-3(CAS DataBase Reference) | [Storage Precautions]
Moisture sensitive;Store under inert gas;Air sensitive |
| Safety Data | Back Directory | [Hazard Codes ]
Xn | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin .
R22:Harmful if swallowed. | [Safety Statements ]
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice . | [WGK Germany ]
3
| [F ]
10-13-23 | [TSCA ]
No | [HazardClass ]
AIR SENSITIVE | [HS Code ]
29310099 |
| Hazard Information | Back Directory | [Description]
2-(Dicyclohexylphosphino)biphenyl is a ligand for the amination of triflates and aryl halides. Optimal ligand for a novel amination reaction (Buchwald reaction)(2) 2-(Dicyclohexylphosphino)biphenyl, a synthetic chemical compound, has found diverse applications in scientific research. Being a cyclic phosphonate derivative of cyclohexanol, this colourless, odourless, and tasteless solid holds a distinct appeal for laboratory use. In scientific research, 2-(Dicyclohexylphosphino)biphenyl has proven invaluable. It serves as an excellent model compound for delving into protein-ligand interactions, thus aiding in studying small molecule effects on protein folding. Moreover, researchers have utilized 2-(Dicyclohexylphosphino)biphenyl to gain insights into protein structures and unravel the mechanism of action behind various drugs. Additionally, the compound has been a vital tool in assessing how environmental factors influence protein structure and function. The interaction between 2-(Dicyclohexylphosphino)biphenyl and proteins primarily occurs through hydrogen bonding and hydrophobic interactions, resulting in high-affinity and specific protein binding. | [Chemical Properties]
white to light yellow crystal powde | [Uses]
Ligand employed in an extremely general method for the Pd-catalyzed synthesis of aromaticamines using aryl chlorides, bromides and triflates. | [Uses]
suzuki reaction | [General Description]
CyJohnPhos [(2-Biphenyl)dicyclohexylphosphine] is an air-stable, bulky and electron-rich monodentate biarylphosphine ligand developed by the Buchwald group to enhance the reactivity of palladium catalysis during cross-coupling reactions. | [reaction suitability]
reaction type: Cross Couplings
reagent type: ligand
reaction type: Arylations
reagent type: ligand
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reagent type: ligand
reaction type: C-X Bond Formation
reagent type: ligand
reaction type: Hiyama Coupling
reagent type: ligand
reaction type: Methylations
reagent type: ligand
reaction type: Negishi Coupling
reagent type: ligand
reaction type: Oxidations
reagent type: ligand
reaction type: Suzuki-Miyaura Coupling |
| Questions And Answer | Back Directory | [Reaction]
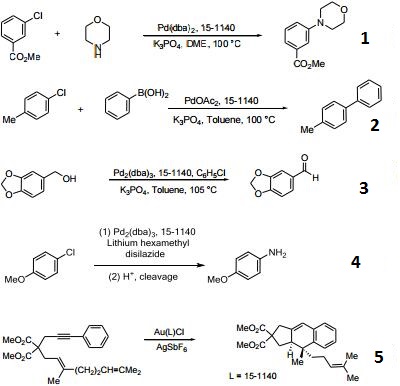
- Ligand used in the palladium-catalyzed synthesis of aromatic amines from aryl chlorides, bromides and triflates.
- Ligand employed in Suzuki coupling reactions involving aryl chlorides, bromides and triflates.
- Useful ligand for the Pd-catalyzed oxidation of alcohols in the presence of chlorobenzenes.
- Useful ligand for the Pd-catalyzed amination with ammonia equivalents.
- Ligand for the gold(I)-catalyzed intramolecular [4+2] cycloadditions involving 1,3-enynes and arylalkynes with alkenes.
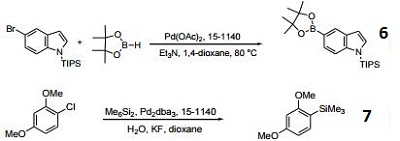
- Ligand used in the palladium-catalyzed borylation of aryl bromdies.
- Ligand used in the palladium-catalyzed siliylation of aryl chlorides.
|
|
|