| Identification | More | [Name]
2,3-Dichloroquinoxaline | [CAS]
2213-63-0 | [Synonyms]
2,3-DICHLOROQUINOXALINE
AKOS BBS-00006726
SALOR-INT L497169-1EA
TIMTEC-BB SBB003555
2,3-dichloro-quinoxalin
cp42103-4
2,3-Dichloroquinoxaline,96%
Quinoxaline, 2,3-dichloro.
2,3-Dichloroquinoxaline ,98% | [EINECS(EC#)]
218-667-3 | [Molecular Formula]
C8H4Cl2N2 | [MDL Number]
MFCD00006720 | [Molecular Weight]
199.04 | [MOL File]
2213-63-0.mol |
| Chemical Properties | Back Directory | [Appearance]
solid | [Melting point ]
152-154 °C (lit.) | [Boiling point ]
325.69°C (rough estimate) | [density ]
1.4994 (rough estimate) | [refractive index ]
1.6400 (estimate) | [storage temp. ]
Keep in dark place,Sealed in dry,Room Temperature | [solubility ]
soluble in Chloroform | [Water Solubility ]
Insoluble in water | [form ]
Needles | [pka]
-4.78±0.48(Predicted) | [color ]
Off-white to light brown | [Stability:]
Stable. Incompatible with strong oxidizing agents. | [Detection Methods]
GC,NMR | [BRN ]
126076 | [InChIKey]
SPSSDDOTEZKOOV-UHFFFAOYSA-N | [CAS DataBase Reference]
2213-63-0(CAS DataBase Reference) | [EPA Substance Registry System]
2213-63-0(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
T | [Risk Statements ]
R25:Toxic if swallowed.
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) .
S37/39:Wear suitable gloves and eye/face protection . | [RIDADR ]
2811 | [WGK Germany ]
3
| [RTECS ]
VD1720000
| [TSCA ]
Yes | [HazardClass ]
6.1 | [PackingGroup ]
III | [HS Code ]
29339900 | [Toxicity]
mouse,LD50,intravenous,5600ug/kg (5.6mg/kg),U.S. Army Armament Research & Development Command, Chemical Systems Laboratory, NIOSH Exchange Chemicals. Vol. NX#03256, |
| Raw materials And Preparation Products | Back Directory | [Preparation Products]
2 CHLORO-3-AMINO QUINOXALINE-->pilaralisib-->2-CHLORO-3-(3,5-DIMETHYL-PYRAZOL-1-YL)-QUINOXALINE-->1,2,3,4,4A,5-HEXAHYDROPYRIDO[1',2':4,5][1,4]OXAZINO[2,3-B]QUINOXALINE-->2-CHLORO-3-(4-MORPHOLINYL)QUINOXALINE-->12H-quinoxalino[2,3-b][1,4]benzoxazine-->2-Chloro-3-ethoxy-quinoxaline-->N-(4-{[(3-CHLOROQUINOXALIN-2-YL)AMINO]SULFONYL}PHENYL)ACETAMIDE-->NSC42848-->2-Chloro-3-(1H-indol-3-yl)-quinoxaline, 98+% C16H10ClN3, MW: 279.73-->2-N,3-N-diphenylquinoxaline-2,3-diamine-->2-chloro-3-hydrazinylquinoxaline-->Quinoxaline, 2,3-dihydrazinyl--->Quinoxaline, 2-chloro-3-(2-naphthalenyl)- |
| Hazard Information | Back Directory | [General Description]
Gray solid. Insoluble in water. | [Reactivity Profile]
A halogenated amine. Amines are chemical bases. They neutralize acids to form salts plus water. These acid-base reactions are exothermic. The amount of heat that is evolved per mole of amine in a neutralization is largely independent of the strength of the amine as a base. Amines may be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen is generated by amines in combination with strong reducing agents, such as hydrides. | [Air & Water Reactions]
Insoluble in water. | [Fire Hazard]
This chemical is probably combustible. | [Description]
2,3-Dichloroquinoxaline (DCQX) can be used as a substrate for nucleophilic aromatic substitution (SNAr) reactions and is a versatile building block for the synthesis of many quinoxaline derivatives. 2,3-Dichloroquinoxaline reacts with 6-aminothiouracil in ethanol/TEA to form 6-amino-2-(3-chloroquinoxalin-2-ylthio)pyrimidin-4(3H)-one. It reacts with cholest-5-en-3-one semicarbazone/thiosemicarbazone to form steriodal cholest-5-en-3-oxazolo and thiazoloquinoxaline. | [Chemical Properties]
solid | [Uses]
2,3-Dichloroquinoxaline is a dichlorinated quinoxaline derivative with antimicrobial activity. 2,3-Dichloroquinoxaline is used in the preparation of quinoxaline derivatives of cancer chemopreventive a
ctivity. | [Preparation]
To a stirred solution of quinoxaline-2,3(1H,4H)-dione (5.00 g, 1.0 equiv.), POCl3 was added (20 ml) and refluxed at 100 °C for 3h. After completion of the reaction, as indicated by TLC, the reaction mass was distilled under vaccum and quenched with ice cold water. Off white semi solid was formed and is filtered through buchner funnel under vaccum to yield the 2,3-dichloroquinoxaline in excellent yield.
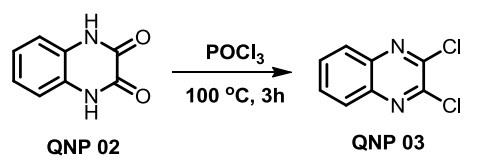
Appearance: off white solid; Yield: 92% (5.64g); ESI-MS: (m/z) calcd. for C8H4Cl2N2: 197.98, found: 199.10 [M+H]+. | [Purification Methods]
Recrystallise it from *C6H6 and dry it in a vacuum [Cheeseman J Chem Soc 1804 1955, Beilstein 23/7 V 144]. |
|
|