| Identification | Back Directory | [Name]
Dimethylammoniumdichlorotri(mu-chloro)bis[(S)-(-)-2,2'-bis(diphenylphosphino)-1,1'-binaphthyl]diruthenate(II) | [CAS]
199541-17-8 | [Synonyms]
-bis(diphenylphosphino)-1,1'
(S)-[(RuCl(BINAP))2(μ-Cl)3][NH2Me2]
[NH2Me2][{RuCl((S)-binap)}2(mu-Cl)3]
(S)-[(RuCl(BINAP))2(mu-Cl)3][NH2Me2]
(S)-[(RuCl(BINAP))2(μ
-binaphthyl]diruthenate(II),[NH2Me2][{RuCl((S)
-Cl)3][NH2Me2]
Dimethylammonium dichlorotri(μ-chloro)bis[(S)-(-)-2,2'
Dimethylammoniumdichlorotri(mu-chloro)bis[(S)-(-)-2,2'-bis(diphenylphosphino)-1,1'-binaphthyl]diruthenate(II)
Dimethylammonium dichlorotri(μ-chloro)bis[(S)-(-)-2,2μ-bis(diphenylphosphino)-1,1μ-binaphthyl]diruthenate(II)
Dimethylammonium dichlorotri(chloro)bis[(S)-(-)-2,2'-bis(diphenylphosphino)-1,1'-binaphthyl]diruthenate(II) [NH2Me2][{RuCl((S)-binap)}2(Cl)3]
DiMethylaMMoniuM dichlorotri(Mu-chloro)bis[(S)-(-)-2,2'-bis(diphenylphosphino)-1,1'-binaphthyl]diruthenate(II) [NH2Me2][{RuCl((S)-binap)}2(μ-Cl)3] | [Molecular Formula]
2C44H32P2.C2H7N.Cl.Cl3Ru2.ClH | [MDL Number]
MFCD09753033 | [MOL File]
199541-17-8.mol | [Molecular Weight]
1670.87 |
| Chemical Properties | Back Directory | [Melting point ]
>100°C | [storage temp. ]
2-8°C | [Water Solubility ]
Insoluble in water | [form ]
Powder | [color ]
orange | [Sensitive ]
air sensitive |
| Questions And Answer | Back Directory | [Reaction]
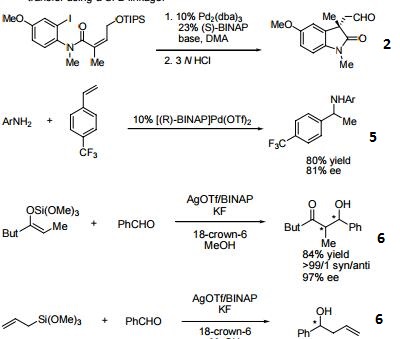
- (R)-BINAP or (R)-Tol-BINAP can be combined with dichloro(1,5-cyclooctadiene)ruthenium to form precursors to NOYORI CATALYST SYSTEMS. These systems exhibit very high catalytic activity and enantioselectivity in the hydrogenation of a wide range of substrates. NOYORI CATALYST SYSTEMS have been shown to effect highly enantioselective hydrogenation of functionalized ketones where the substituents are dialkylamino, hydroxy, siloxy, carbonyl, ester, amide or thioester.
- Useful ligand in asymmetric Heck processes.
- 3. Ligand employed in palladium-catalyzed asymmetric arylation of ketones.
- 4. Ligand employed in rhodium-catalyzed 1,4-additions to enones.
- 5. Ligand employed in palladium-catalyzed hydroamination of styrene derivatives.
- 6. Ligand employed in silver-catalyzed asymmetric Sakuri-Hosomi allylation and Mukaiyama aldol reaction.
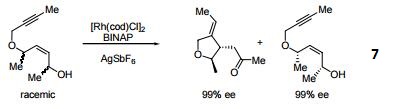
- 7. Ligand employed in rhodium-catalyzed kinetic resolution of enynes.
- 8. Ligand employed in asymmetric rhodium-catalyzed hydroboration of cyclopropenes.
- 9. Ligand employed in silver-catalyzed a-hydroxylation of stannyl enol ethers.
- 10. Ligand employed in palladium-catalyzed synthesis of chiral allenes.
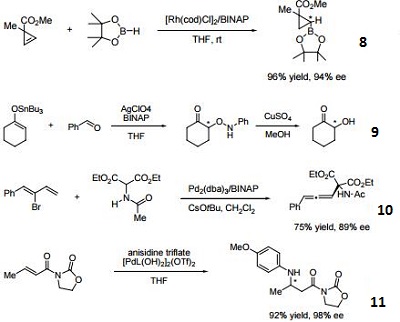
- 11. Ligand for palladium-catalyzed enantioselective hetero Michael addition to form b-amino acid derivatives.
- 12. Ligand employed in rhodium-catalyzed asymmetric rearrangement of alkynyl alkenyl carbinols.
- 13. Ligand employed in rhodium-catalyzed 1,2-addition of aluminium organyl compounds to cyclic enones.
- 14. Ligand employed in iridium-catalyzed transfer hydrogenative allylation of benzylic alcohols.
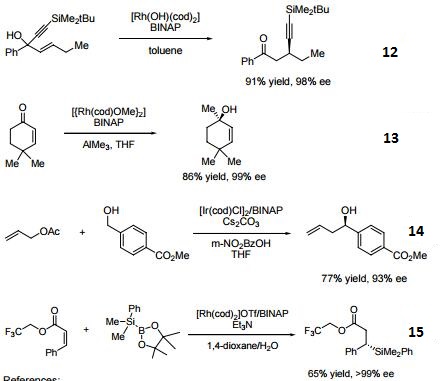
- 15. Ligand employed in rhodium-catalyzed asymmetric C-Si bond formation by conjugate silyl transfer using a Si-B linkage.
|
|
| Company Name: |
Energy Chemical
|
| Tel: |
021-021-58432009 400-005-6266 |
| Website: |
http://www.energy-chemical.com |
| Company Name: |
TCI Europe
|
| Tel: |
320-37350700 |
| Website: |
https://www.tcichemicals.com/de/de/index.html |
| Company Name: |
TCI AMERICA
|
| Tel: |
800-4238616 |
| Website: |
https://www.tcichemicals.com/en/us/index.html |
| Company Name: |
Sigma-Aldrich
|
| Tel: |
021-61415566 800-8193336 |
| Website: |
https://www.sigmaaldrich.cn |
| Company Name: |
LaaJoo
|
| Tel: |
021-60702684 18516024827 |
| Website: |
http://www.approvedhomemanagement.com/ShowSupplierProductsList20079/0.htm |
|