| Identification | More | [Name]
4-Butylresorcinol | [CAS]
18979-61-8 | [Synonyms]
2,4-DIHYDROXY-N-BUTYL BENZENE
4-N-BUTYLRESORCINOL
2,4-DIHYDROXY-N-BUTYL BENZEN
2,4-Dihydroxy-n-bytyl benzen
4-06-00-06003 (Beilstein Handbook Reference)
4-BUTYLRESORCINOL,98+%
4-Butylbenzene-1,3-diol
4-Butylresorcinol | [EINECS(EC#)]
606-191-2 | [Molecular Formula]
C10H14O2 | [MDL Number]
MFCD01684800 | [Molecular Weight]
166.22 | [MOL File]
18979-61-8.mol |
| Chemical Properties | Back Directory | [Melting point ]
50.0 to 55.0 °C | [Boiling point ]
166°C/7mmHg(lit.) | [density ]
1.092±0.06 g/cm3(Predicted) | [vapor pressure ]
0.041Pa at 25℃ | [storage temp. ]
Inert atmosphere,Room Temperature | [solubility ]
DMSO : 150 mg/mL (902.42 mM) | [form ]
Powder | [pka]
9.95±0.18(Predicted) | [color ]
white to pale yellow powder | [Odor]
Characteristic | [InChI]
InChI=1S/C10H14O2/c1-2-3-4-8-5-6-9(11)7-10(8)12/h5-7,11-12H,2-4H2,1H3 | [InChIKey]
CSHZYWUPJWVTMQ-UHFFFAOYSA-N | [SMILES]
C1(O)=CC=C(CCCC)C(O)=C1 | [LogP]
2.8 at 24℃ and pH7 | [Surface tension]
17.8mN/m at 1g/L and 20℃ | [CAS DataBase Reference]
18979-61-8(CAS DataBase Reference) |
| Questions And Answer | Back Directory | [resorcinol derivative]
4-n-Butylresorcinol is a resorcinol derivative that inhibits both tyrosinase and tyrosinase-related protein-1 (TRP-1). It may be used to decrease skin irritation and is also known to inhibit melanin production. Its hypopigmenting action was first reported in 1995, and many following studies have documented its efficacy and safety in melasma treatment with the 0.1% cream, but there is paucity of clinical studies that used the 0.3% cream. The biochemical assay on inhibition of human tyrosinase activity has revealed the superiority of 4-n-butylresorcinol over other hypopigmenting agents. Many studies show good efficacy and safety in treating melisma.

4-n-Butylresorcinol may be used to produce a safe cosmetic agent, with the clinical efficacy required of a pharmacological agent. Furthermore, 4-n-butylresorcinol acts mainly by inhibition of tyrosinase activity and has no effect on MITF. 4-n-butylresorcinol showed an additive effect in combination with hinokitiol, which reduces MITF expression.
| [History]
4-n-Butylresorcinol (Rucinol® ) (Obtained by POLA in 1998) was selected by screening synthetic resorcinol derivatives that can elicit strong competitive inhibition of tyrosinase activity. Melanin synthesis is catalyzed by tyrosinase, together with tyrosinase-related proteins (TRP) -1 and -2, and Rucinol® has been shown to inhibit melanin synthesis in cultured mouse melanocytes via direct inhibition not only of tyrosinase activity, but also of TRP-1 activity. A 0.3% Rucinol® -containing lotion was shown to be effective for treating hyperpigmentary disorders, such as melasma.
| [Uses]
4-Butylresorcinol(18979-61-8) is considered a potential Cytochrome P450 inhibitor. It can cause hypopigmentation due to its direct inhibition of tyrosinase.
| [tyrosinase inhibitor]
4-butylresorcinol(18979-61-8) is use as an effective treatment option for topical hyperpigmentation management. Similar to hydroquinone, 4-n-butylresorcinol is also a tyrosinase inhibitor. It has been characterized as a strong tyrosinase25 and TRP‐126 inhibitor. We measured an IC50 in the human tyrosinase assay of 21μmol/L for 4‐butylresorcinol compared with 94 and 131?μmol/L for 4‐hexylresorcinol and 4‐phenylethylresorcinol respectively. Also on skin models, 4‐butylresorcinol was most effective of all tested substances with an IC50 of 13.5 μmol/L. Therefore, 4‐butylresorcinol was selected for several clinical studies to prove in vivo efficacy. In comparison with 4‐hexylresorcinol and 4‐phenylethylresorcinol, 4‐butylresorcinol treated age spots showed a faster onset of improvement and also a higher degree of lightening after 12 weeks of treatment.
|
| Hazard Information | Back Directory | [Description]
4-n-butylresorcinol(18979-61-8) is a derivative of resorcinol and a potent human tyrosinase inhibitor. It may be used to decrease skin irritation and is also known to inhibit melanin production.
| [Application]
Glycerol is used both in sample preparation and gel formation for polyacrylamide gel electrophoresis. Glycerol (5-10%) increases the density of a sample so that the sample will layer at the bottom of a gel′s sample well. Glycerol is also used to aid in casting gradient gels and as a protein stabilizer and storage buffer component. | [Definition]
ChEBI: 4-n-Butylresorcinol is a member of resorcinols. | [Biological Activity]
Glutathione reductase IGR) is a crucial flavoenzyme in the antioxidant defense system. Reduced glutathione (GSH) is used by glutathione peroxidase to detoxify hydrogen peroxide and in the process is converted to oxidized glutathione (GSSG). The GSSG is then recycled back to GSH by glutathione reductase (GR) using NADPH that is then converted to NADP+. The regenerated GSH is then available to detoxify more hydrogen peroxide. The enzyme uses FAD as a cofactor. GR and glutathione peroxidase may inhibit lipid peroxidation by functioning as antioxidant enzymes in sperm. Glutathione reductase shares a structural motif with a number of other proteins including aspartyl proteases, citrate synthase, EF hands, hemoglobins, lipocalins, and α/β hydrolases. GR is stimulated by melatonin and is reportedly irreversibly inhibited by a number of oxygen radical generating systems.A sweet tastant for mammals. A glycerol taste receptor binding site specific for glucose has been proposed in drosophila. | [Mechanism of action]
The mechanism of action of 4-butylresorcinol depigmentation is through inhibition of tyrosinase and tyrosinase-related protein-1 (TRP-1)[1]. | [Synthesis]
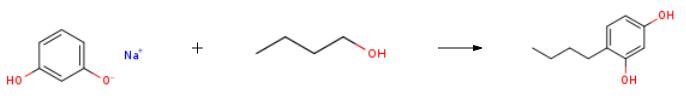
Under the protection of nitrogen, 110 g (1 mol) of resorcinol and 220 g of n-heptane were mixed in the reaction flask, heated and dissolved, and the temperature was lowered to 10 ?? C. A turbid solution of 40.8 g (1.02 mol) of sodium hydroxide and 100 g of n-butanol was added in portions. , And control the temperature of 10 to 15 , after TLC (EA developing agent) detection of almost no remaining raw materials, add n-butanol (2.5mol), tris (pentafluorobenzene) borane 17.5g (0.1mol) and calcium chloride 1.1 g, raise the temperature to 40-45 ?? C for 1 hour, then raise the temperature to 98 ?? C for reflux and water separation. After 8 hours of water separation, take a sample and quench the GC to detect resorcinol <1%, cool to room temperature, add a small amount of water to quench It is extinguished, and then dilute sulfuric acid is added to adjust the pH = 1-2. The insoluble solids are filtered through diatomaceous earth, and the organic phase is concentrated to a stagnant liquid. Water is added for replacement, and then 660 g of water, 2.2 g of sodium thiosulfate, and 5.5 g are added. Activated carbon was heated to reflux for 1 hour, and was hot-filtered to obtain a pale yellow solution. Then, flaky crystals were precipitated by cooling, and 151 g of 4-n-butylresorcinol was obtained by filtration, GC: 99.3%, and yield: 90.9%. | [References]
[1] D. RESENDE. Skin Depigmenting Agents in Anti-Aging Cosmetics: A Medicinal Perspective on Emerging Ingredients[J]. Applied Sciences-Basel, 2022. DOI:10.3390/app12020775. |
|
|