| Identification | More | [Name]
CHLOROMETHYLDIMETHYLPHENYLSILANE | [CAS]
1833-51-8 | [Synonyms]
CHLOROMETHYLDIMETHYLPHENYLSILANE
(chloromethyl)dimethylphenyl-silan
Dichloromethyldimethylphenylsilane
Phenyldimethyl(chloromethyl)silane
Silane, (chloromethyl)dimethylphenyl-
Chloromethylphenyldimethylsilane
Phenyl(chloromethyl)dimethylsilane
Dimethylphenylsilyldichloromethane | [EINECS(EC#)]
217-392-6 | [Molecular Formula]
C9H13ClSi | [MDL Number]
MFCD00040858 | [Molecular Weight]
184.74 | [MOL File]
1833-51-8.mol |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36:Wear suitable protective clothing . | [RIDADR ]
1993 | [WGK Germany ]
3
| [TSCA ]
Yes | [HS Code ]
29319000 |
| Hazard Information | Back Directory | [Physical properties]
bp 106–107°C/15 mmHg; d 1.024 g cm?3 | [Uses]
The first reported preparation and use of
(chloromethyl)dimethylphenylsilane was in 1949.At the time,
the main utility of (chloromethyl)dimethylphenylsilane was as
the starting material for conversion to the corresponding Grignard
reagent (see below). Since then, it has also been used for heteroatom
alkylation, carbon alkylation, and conversion to a variety
of organometallic and organolanthanide reagents. The main advantage
of this reagent over the closely related (chloromethyl)trimethylsilane
is the ability of (chloromethyl)dimethylphenylsilane
to undergo a Fleming oxidation, thus allowing (chloromethyl)dimethylphenylsilane
to serve as a masked hydroxyl group. This
utility has been exploited for both the C-substituted and Nsubstituted
adducts. A major disadvantage of the use of
(chloromethyl)dimethylphenylsilane is its propensity to undergo
rearrangements under a variety of conditions (see below). (Chloromethyl)
dimethylphenylsilane is used as the precursor for the
preparation of (phenyldimethylsilyl)methoxymethyl chloride
(SMOM-Cl), a hydroxyl protecting group.
(Chloromethyl)dimethylphenylsilane (1) can
be utilized to install a silylmethyl group on carbon via base promoted
C-alkylation of terminal alkynes,dihydropyrazines,malonic
esters,phenylacetonitriles,sulfoxides,and imines.Although
has been used directly in the alkylation, conversion to
the corresponding iodide via Finkelstein displacement (eq 1)
prior to alkylation is sometimeswarranted. Except for the malonic
esters (eq 2), strongly basic conditions and lowtemperatures (with
slow warming) are generally employed in the transformation (eqs
3 and 4).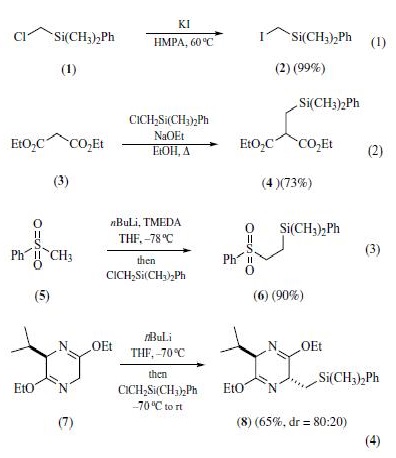
| [Preparation]
can be prepared either by the reaction
of phenylmagnesium bromide with chloro(chloromethyl)dimethylsilane
in ether at reflux,or by the reaction of phenylmagnesium
bromide with chloro(chloromethyl)dimethylsilane
in the presence of catalytic (N,N,N�,N�-tetramethylethylenediamine)
zinc in 1,4-dioxane at 20°C. |
|
|