| Identification | More | [Name]
Cephalexin | [CAS]
15686-71-2 | [Synonyms]
(6R,7R)-7-((R)-2-AMINO-2-PHENYL-ACETYLAMINO)-3-METHYL-8-OXO-5-THIA-1-AZA-BICYCLO[4.2.0]OCT-2-ENE-2-CARBOXYLIC ACID
7-(D-ALPHA-AMINO-PHENYLACETAMIDO)-3-METHYL-3-CEPHEME-4-CARBOXYLIC ACID
CEFALEXIN
CEFALEXIN SODIUM
CEPHALEXIN
CEPHALEXINE
CEPOREX
CEPOREXINE
(6r-(6alpha,7beta(r*)))-ino)-3-methyl-8-oxo
5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylicacid,7-((aminophenylacetyl)am
5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylicacid,7-(2-amino-2-phenylaceta
7-(d-2-amino-2-phenylacetamido)-3-methyl-delta(sup3)-cephem-4-carboxylica
7-(d-2-amino-2-phenylacetamido)-3-methyl-delta3-cephem-4-carboxylicacid
7-(d-alpha-aminophenylacetamido)desacetoxycephalosporanicacid
7-beta-(d-alpha-amino-alpha-phenylacetylamino)-3-methyl-3-cephem-4-carboxyli
cefa-iskia
cefaloto
ceporexin
cex
d-mido)-3-methyl-8-oxo | [EINECS(EC#)]
239-773-6 | [Molecular Formula]
C16H17N3O4S | [MDL Number]
MFCD04966776 | [Molecular Weight]
347.39 | [MOL File]
15686-71-2.mol |
| Safety Data | Back Directory | [Hazard Codes ]
Xn | [Risk Statements ]
R42/43:May cause sensitization by inhalation and skin contact . | [Safety Statements ]
S22:Do not breathe dust .
S36/37:Wear suitable protective clothing and gloves .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) . | [WGK Germany ]
3
| [RTECS ]
XI0350000 | [HS Code ]
29419059 | [Hazardous Substances Data]
15686-71-2(Hazardous Substances Data) | [Toxicity]
TDLo orl-hmn: 14 mg/kg/D:GIT AACHAX -,361,68 |
| Questions And Answer | Back Directory | [Description]
Cephalexin is a cephalosporin antibiotic that is used to examine the impact of binding, expression, and inhibition of PBP3 as well as additional penicillin-binding proteins (PBPs) on the cell wall during bacterial mucopeptide synthesis. Cephalexin is used for the treatment of infection-causing bacteria that may induce ear, respiratory, urinary tract, and skin infections. Bacteria that are defenseless against Cephalexin may include Streptococcus pneumonia, Staphylococcus aureus, E. coli, and Haemophilus influenza. Cephalexin is also referred to as Keflex (brand name), and it does not relieve viral infections such as flu or colds.
| [Indications]
Cephalexin is prescribed for the treatment of otitis media, genitourinary, bone, respiratory, and skin structure infections.
| [Mechanism of Action]
The mechanism of action of Cephalexin resembles that of penicillin where it inhibits synthesis of the bacterial cell wall, its absence influences death as a result of bacterial lysis. Cell lysis is further mediated by autolytic enzymes particular to the bacterial cell wall, which includes autolysis. Research indicates that there is a probability that Cephalexin impedes the functionality of an autolysin inhibitor.
| [Brand Name(s) in US]
Keflex and generic forms
| [Pharmacodynamics]
Cephalexin is a 1st generation cephalosporin antibiotic that is widely prescribed for the treatment of external infections that may arise from complications associated with lacerations or minor wounds. The drug is effective in fighting a majority of gram-positive bacteria.
Cephalexin illustrates vitro activity that opposes methicillin-susceptible Staphylococcus aureus, a notable pathogen in osteoarticular infections. However, pharmacodynamics and pharmacokinetics are inadequately defined in children.
| [Interactions]
Cephalexin may minimize the impact of typhoid and BCG vaccines. Notably, these three drugs should not be administered at the same time. Patients are also advised to take the drug on an empty stomach at least 1-2 hours after meals.
| [Uses]
Cephalexin is administered to minimize the development of bacteria that are resistant to drugs. To maintain the overall effectiveness of Cephalexin, the drug should be prescribed as a treatment for infections that can be attributed to bacteria. The availability of susceptibility and culture information should be put into consideration while making modifications to antibacterial therapy. The absence of such information may be backed by susceptibility and epidemiology patterns to influence verifiable adoption of treatment.
In some cases, Cephalexin is used for the treatment of patients who are allergic to penicillin and may have a heart condition at the time when they are undergoing a procedure on their respiratory tract, to inhibit the development of infection on their heart valves.
| [Dosing Information]
A standard dose of Cephalexin should be administered orally in 250mg every 6 hours. Alternatively, in 12 hours, a dose of 500mg should be administered to the patient during the 7-14days treatment period. In instances where the infections are severe, higher doses up to 4g should be administered in 2-4 equal does every day.
For pediatric patients, the appropriate daily dose of Cephalexin is 25-40mg/kg administered in equal doses for a period of 7-14days. Severe infections may necessitate 50-100mg/kg administered in equal doses.
The treatment of Otitis media necessitates 75-100mg/kg in equal doses of Cephalexin. For patients with renal impairments, the dosage requirements may be adjusted accordingly for both pediatric and adult patients.
| [Elimination]
Cephalexin undergoes tubular secretion and glomerular filtration before it is eliminated in urine. Studies indicate that about 90% of Cephalexin is eliminated in its unaltered form in urine within 8 hours.
| [Side effects]
Allergic reactions to Cephalexin may result in respiratory issues, swelling of the tongue, lips, face, or the throat, and hives. Nonetheless, one may need to consult a doctor if they experience watery diarrhea or intense stomach pains, unusual bleeding or easy bruising, minimal or no urination, hallucinations, confusion or agitation, and severe skin reaction.
Common side effects associated with Cephalexin include vaginal discharge or itching, skin rash, fever, nausea, vomiting, joint pain, headache, feeling of exhaustion, dizziness, or diarrhea.
| [Safety Precautions]
A patient should inform the pharmacist or doctor of any allergic reactions to Cephalexin, associated cephalosporin antibiotics, and additional ingredients or medications. The patient should also indicate any nutritional supplements, vitamins, herbal products or medications they are taking or they are planning to take. It is important to notify the doctor of a preexisting liver, kidney or gastrointestinal disease, especially colitis, if one is either pregnant or planning on getting pregnant, and if they conceive while taking Cephalexin.
If Cephalexin is prescribed to a patient with no clear indications of a bacterial infection, there are minimal chances that the drug will benefit the patient. Instead, it will increase the patient’s chances of developing drug-resistant bacteria.
Using Cephalexin over extended periods of time may induce the overgrowth of non-susceptible organisms. Doctors should examine their patients for superinfections during therapy for the implementation of appropriate treatment measures.
|
| Hazard Information | Back Directory | [Chemical Properties]
White cryst. powder | [Originator]
Ceporex,Glaxo,UK,1970 | [Definition]
ChEBI: A semisynthetic first-generation cephalosporin antibiotic having methyl and beta-(2R)-2-amino-2-phenylacetamido groups at the 3- and 7- of the cephem skeleton, respectively. It is effective against both Gram-negative and G
am-positive organisms, and is used for treatment of infections of the skin, respiratory tract and urinary tract. | [Manufacturing Process]
To a 1 liter flask containing dimethylformamide at 0°C, was added 24.8 g
sodium N-(2-methoxycarbonyl-1-methylvinyl)-D-α-phenylglycine (prepared
from sodium D-α-phenylglycine and methyl acetoacetate). The mixture was
cooled to -40°C and methyl chloroformate (7.5 ml) and dimethylbenzylamine
(0.26 ml) added. After stirring for 25 minutes, p-nitrobenzyl 7-
aminodesacetoxycephalosporanate (32.8 g) in the form of its hydrochloride
salt was added, followed by triethylamine (12.1 ml) and dimethylformamide
(140 ml) over a period of 20 minutes. The reaction mixture was stirred for 2
hours at -25°C to -35°C, then warmed to 0°C and water (32 ml) added. To
the resultant solution, hydrochloric acid (54 ml) was added followed by zinc
(21.8 g) in portions over a period of 5 minutes, the temperature being
maintained at 5°C to 10°C. Further hydrochloric acid (35 ml) was added and
the solution stirred at 15°C to 20°C for 7 hours.
The pH was adjusted to 3.3 with triethylamine and
semicarbazidehydrochloride (9.5 g) added. The mixture was brought back to
pH 3 with further triethylamine, then stirred for 30 minutes at pH 3. The
resultant mixture was adjusted slowly over 4 hours to pH 6.8 by addition of
triethylamine, seeding being carried out when pH 4.5 was reached. The precipitated cephalexin was filtered off, washed with dimethylformamide (200
ml) and the cephalexin recovered, yield 75%. | [Brand name]
Keflex (Panixine (Ranbaxy). | [Therapeutic Function]
Antibiotic | [Antimicrobial activity]
It is resistant to staphylococcal β-lactamase. Gram-positive
rods and fastidious Gram-negative bacilli, such as Bordetella
spp. and H. influenzae, are relatively resistant. It is active
against a range of enterobacteria, but it is degraded by
many enterobacterial β-lactamases. Citrobacter, Edwardsiella,
Enterobacter, Hafnia, Providencia and Serratia spp. are all
resistant. Gram-negative anaerobes other than B. fragilis are
susceptible. Because of its mode of action it is only
slowly bactericidal to Gram-negative bacilli. | [Pharmacokinetics]
Oral absorption: >90%
Cmax 500 mg oral: c. 10–20 mg/L after 1 h
Plasma half-life: 0.5–1 h
Volume of distribution: 15 L
Plasma protein binding: 10–15%
Absorption and distribution
It is almost completely absorbed when given by mouth, the
peak concentration being delayed by food. Intramuscular
preparations are not available: injection is painful and produces
delayed peak plasma concentrations considerably lower
than those obtained by oral administration.
In synovial fluid, levels of 6–38 mg/L have been described
after a 4 g oral dose, but penetration into the CSF is poor.
Useful levels are achieved in bone (9–44 mg/kg after 1 g orally)
and in purulent sputum. Concentrations of 10–20 mg/L have
been found in breast milk. Concentrations in cord blood
following a maternal oral dose of 0.25 g were minimal.
Metabolism and excretion
It is not metabolized. Almost all the dose is recoverable from
the urine within the first 6 h, producing urinary concentrations
exceeding 1 g/L. The involvement of tubular secretion
is indicated by the increased plasma peak concentration and
reduced urinary excretion produced by probenecid. Renal
clearance is around 200 mL/min and is depressed in renal
failure, although a therapeutic concentration is still obtained
in the urine. It is removed by peritoneal and hemodialysis.
Some is excreted in the bile, in which therapeutic concentrations
may be achieved. | [Clinical Use]
As for group 2 cephalosporins . It should not be used
in infections in which H. influenzae is, or is likely to be, implicated.
It should not be used as an alternative to penicillin in
syphilis. | [Clinical Use]
Cephalexin, 7α-(D-amino-α-phenylacetamido)-3-methylcephemcarboxylicacid (Keflex, Keforal), was designed purposelyas an orally active, semisynthetic cephalosporin. Theoral inactivation of cephalosporins has been attributed to twocauses: instability of the β-lactam ring to acid hydrolysis(cephalothin and cephaloridine) and solvolysis or microbialtransformation of the 3-methylacetoxy group (cephalothin,cephaloglycin). The α-amino group of cephalexin renders itacid stable, and reduction of the 3-acetoxymethyl to a methylgroup circumvents reaction at that site.
Cephalexin occurs as a white crystalline monohydrate. Itis freely soluble in water, resistant to acid, and absorbed wellorally. Food does not interfere with its absorption. Becauseof minimal protein binding and nearly exclusive renal excretion,cephalexin is recommended particularly for the treatmentof urinary tract infections. It is also sometimes used forupper respiratory tract infections. Its spectrum of activity isvery similar to those of cephalothin and cephaloridine.Cephalexin is somewhat less potent than these two agentsafter parenteral administration and, therefore, is inferior tothem for the treatment of serious systemic infections. | [Safety Profile]
Poison by intraperitoneal route.Moderately toxic by ingestion and other routes. An experimental teratogen. Other experimental reproductiveeffects. Human systemic effects by ingestion: nausea,vomiting, and diarrhea. When heated to decomposition itemits | [Synthesis]
Cephalexin is synthesized from cephalophenylglycine (32.1.2.9),
which is synthesized by reacting 7-aminocephalosporanic acid with a mixed anhydride syn�thesized by reacting N-carbobenzoxyphenylglycine and isobutyl chloroformate in the pres�ence of triethylamine. Removing the N-carbobenzoxy protective group from the resulting
product (32.1.2.8) using hydrogen and a palladium on carbon catalyst gives cephalophenyl�glycine (32.1.2.9) in the form of an internal salt. Reducing this product with hydrogen using
a palladium on barium sulfate catalyst results in the deacetoxylation at the third position of
7-aminocephalosporanic acid, making the desired cephalexin (32.1.2.10).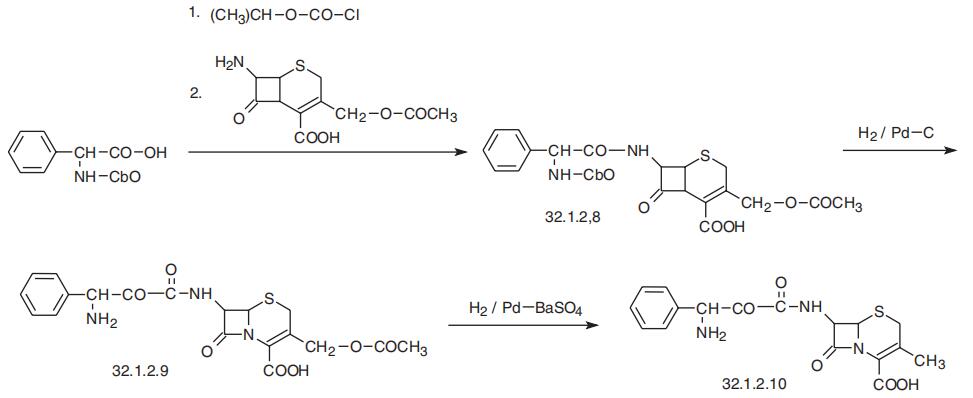
| [Veterinary Drugs and Treatments]
There are no approved cephalexin products for veterinary use in the
USA. However, it has been used clinically in dogs, cats, horses, rabbits,
ferrets, and birds, particularly for susceptible Staphylococcal
infections. | [Drug interactions]
Potentially hazardous interactions with other drugs
Anticoagulants: effects of coumarins may be
enhanced. | [Metabolism]
Cefalexin is not metabolised. About 80% or more of a
dose is excreted unchanged in the urine in the first 6
hours by glomerular filtration and tubular secretion.
Probenecid delays urinary excretion. Therapeutically
effective concentrations may be found in the bile and
some may be excreted by this route. | [storage]
Store at -20°C |
|
|