| Identification | Back Directory | [Name]
2'-C-Methyladenosine | [CAS]
15397-12-3 | [Synonyms]
-C-Methyladenosine
2'-C-Methyladenosine
2'-CMethyl-D-adenosine
2’-β-C-Methyladenosine
Adenosine, 2'-C-methyl-
2'-C-Methyladenosine USP/EP/BP
6-Amino-9-(2'-C-methyl-b-D-ribofuranosyl)purine
2-(6-AMINOPURIN-9-YL)-5-(HYDROXYMETHYL)-3-METHYLOXOLANE-3,4-DIOL
(2R,3R,4R,5R)-2-(6-Amino-9H-purin-9-yl)-5-(hydroxymethyl)-3-methyltetrahydrofuran-3,4-diol | [Molecular Formula]
C11H15N5O4 | [MDL Number]
MFCD02682944 | [MOL File]
15397-12-3.mol | [Molecular Weight]
281.27 |
| Chemical Properties | Back Directory | [Boiling point ]
643.4±65.0 °C(Predicted) | [density ]
1.89 | [storage temp. ]
Store at -20°C | [solubility ]
DMF: 5 mg/mL; DMSO: 20 mg/mL; PBS (pH 7.2): 10 mg/mL | [form ]
A crystalline solid | [pka]
13.18±0.70(Predicted) | [color ]
White to off-white |
| Hazard Information | Back Directory | [Description]
2′-C-Methyladenosine has been shown to inhibit HCV RNA replication in the absence of cytotoxicity. The corresponding triphosphates were shown to be potent, competitive inhibitors of NS5B-catalyzed reactions in vitro. | [Biological Activity]
2’-C-Methyladenosine is an inhibitor of hepatitis C virus (HCV) replication (IC50 = 0.3 μM in Huh-7 human hepatoma cells) that is not cytotoxic at concentrations up to 100 μM.{39652} It is converted intracellularly to adenosine triphosphate, which inhibits the RNA-dependent RNA polymerase nonstructural protein 5B (NS5B). It also inhibits growth of L. guyanensis in vitro (EC50 = 3 μM) and eradicates it when used at a concentration of 10 μM. | [Synthesis]
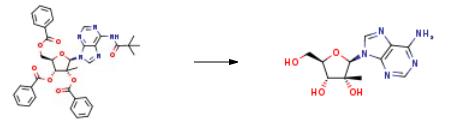
Synthesis of yfl-2'-methyl-adenosine (CHCl); N6-tert-butanoyl-/?-2'-methyl-2',3',5'-tribenzoyl-adenosine (400 mg, 0.590 mmol) was added to a solution of MeOH saturated with ammonia, and stirred at room temperature. After 12 hours the solvent was removed and the obtained solid was purified by column chromatography in gradient starting with a mixture of CHCl3/MeOH 9:1 then 8:2. The pure product was obtained as a white solid (120 mg, 0.427 mmol, 72%). |?H (J6-DMSO): 8.47 (IH, s, H8-adenosine), 8.15 (IH, s, H2-adenosine), 7.30 (IH, s, NH26-adenosine), 5.95 (IH, s, Hl '-adenosine), 5.25-5.21 (3H, m, OH5' -adenosine, OH3'- adenosine, OH2' -adenosine), 4.12-4.05 (IH, d, H3 '-adenosine, J= 8.6 Hz), 3.91 (IH, m, H4'-adenosine), 3.84 (IH, m, H5' -adenosine), 3.70 (IH, m, H5' -adenosine), 0.77 (3H3 s, CH32'-adenosine); |?c (4-DMSO): 156.02 (1C, C6-adenosine), 152.53 (1C, C2-adenosine), 149.01 (1C, C4-adenosine), 138.68 (1C, C8-adenosine), 118.67 (1C, C5-adenosine), 90.78 (1C, Cl '-adenosine), 82.52 (1C, C4' -adenosine), 78.46 (1C, C2'-adenosine), 71.63 (1C, C3' -adenosine), 59.47 (1C, C5 '-adenosine), 19.83 (1C, CH3-2'-adenosine). Anal. CaIc. for C11H15N5O4: C 46.97%, H 5.38%, N 24.90%. Found: C 46.67%, H 5.22%, N 24.20%. | [storage]
Store at -20°C |
|
|