| Identification | More | [Name]
Aminoglutethimide | [CAS]
125-84-8 | [Synonyms]
3-(4-AMINOPHENYL)-3-ETHYL-2,6-PIPERIDINEDIONE
3-(4-AMINOPHENYL)-3-ETHYLPIPERIDINE-2,6-DIONE
3-[P-AMINOPHENYL]-3-ETHYLPIPER-IDINE-2,6-DIONE
AKOS NCG1-0032
AMINOGLUTETHIMIDE
DL-3-(4-AMINOPHENYL)-3-ETHYL-2,6-PIPERIDINEDIONE
DL-AMINOGLUTETHIMIDE
ORIMETEN
(+/-)-P-AMINOGLUTETHIMIDE
(RS)-2-ETHYL-2-(4-AMINO-PHENYL)-GLUTARIMIDE
(RS)-3-(4-AMINO-PHENYL)-3-ETHYL-PIPERIDINE-2,6-DIONE
(RS)-3-ETHYL-3-(4-AMINO-PHENYL)-2,6-DIOXO-PIPERIDINE
TIMTEC-BB SBB000711
2-(p-aminophenyl)-2-ethyl-glutarimid
2-(p-Aminophenyl)-2-ethylglutarimide
2-(p-Aminophenyl)-2-ethylglutarimide2,6-piperidinedione, 3-(4-aminophenyl)-3-ethyl-
2,6-Piperidinedione, 3-(4-aminophenyl)-3-ethyl-
3-(4-aminophenyl)-3-ethyl-6-piperidinedione
3-Ethyl-3-(p-aminophenyl)-2,6-dioxopiperidine
alpha-(p-Aminophenyl)-alpha-ethylglutarimide | [EINECS(EC#)]
204-756-4 | [Molecular Formula]
C13H16N2O2 | [MDL Number]
MFCD00010122 | [Molecular Weight]
232.28 | [MOL File]
125-84-8.mol |
| Chemical Properties | Back Directory | [Appearance]
White Solid | [Melting point ]
152-154 °C(lit.) | [Boiling point ]
374.44°C (rough estimate) | [density ]
1.1099 (rough estimate) | [refractive index ]
1.6450 (estimate) | [storage temp. ]
2-8°C | [solubility ]
H2O: 0.2 mg/mL, slightly soluble
| [form ]
neat | [pka]
11.60±0.40(Predicted) | [color ]
white
| [Water Solubility ]
Soluble in water (2 mg/ml at 20°C), methanol (50 mg/ml), ethanol (7 mg/ml at 25°C), DMSO (20 mg/ml at 25°C), and chloroform. | [Usage]
An aromatase inhibitor. Also blocks adrenal steroidogenesis | [Merck ]
440 | [CAS DataBase Reference]
125-84-8(CAS DataBase Reference) | [NIST Chemistry Reference]
Aminoglutethimide(125-84-8) | [EPA Substance Registry System]
125-84-8(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36:Wear suitable protective clothing . | [RIDADR ]
3249 | [WGK Germany ]
3
| [RTECS ]
MA4026950
| [HazardClass ]
6.1(b) | [PackingGroup ]
III | [HS Code ]
2925190100 | [Hazardous Substances Data]
125-84-8(Hazardous Substances Data) |
| Hazard Information | Back Directory | [Description]
Aminoglutethimide is an aromatase inhibitor (IC50 = 7.5 μM).1 Aromatase inhibitors, including aminoglutethimide, inhibit estrogen synthesis via aromatase, suppressing estrogen levels in post-menopausal women. Formulations containing aromatase inhibitors have been used to treat estrogen receptor-positive breast cancer in post-menopausal women.2,3 | [Chemical Properties]
White Solid | [Originator]
Ellipten,Ciba,US,1960 | [Uses]
Aminoglutethimide is used to decrease the production of sex hormones and suppress the growth of tumors that need sex hormones to grow. It blocks the production of steroids derived from cholesterol and is clinically used in the treatment of Cushing's syndrome and metastatic breast cancer. It is also a drug of abuse by body builders. | [Uses]
An aromatase inhibitor. Also blocks adrenal steroidogenesis | [Uses]
aromatase inhibitor, antineoplastic, testosterone suppressant | [Definition]
ChEBI: A dicarboximide that is a six-membered cyclic compound having ethyl and 4-aminophenyl substituents at the 3-position. | [Indications]
Aminoglutethimide (Cytadren) is a competitive inhibitor
of desmolase, the enzyme that catalyzes the conversion
of cholesterol to pregnenolone; it also inhibits
11-hydroxylase activity.This drug also reduces estrogen
production by inhibiting the aromatase enzyme complex
in peripheral (skin, muscle, fat) and steroid target
tissues. | [Manufacturing Process]
The α-(p-nitrophenyl)-α-ethyl-glutarimide starting material can be prepared as
follows: 217 g of α-phenyl-α-ethyl-glutarimide are dissolved in 800 g of
concentrated sulfuric acid with subsequent cooling to about -10°C and
nitration is carried out at -10°C to +10°C by slow addition of a mixed acid
consisting of 110 g of concentrated sulfuric acid and 110 g of 63% nitric acid.
The nitration solution is stirred into ice, the separated nitro compound taken
up in methylene or ethylene chloride, the solution washed with water and
sodium carbonate solution until neutral and the solvent evaporated under
vacuum. The residue is crystallized from methanol or ethyl acetate, whereby a
yellowish crystal powder of MP 128-136°C is obtained in a yield of about 85%
which consists for the most part of α-(p-nitrophenyl)-α-ethyl-glutarimide. By
recrystallization from methanol the pure p-nitrophenyl compound is obtained
of MP 137-139°C. From the residues of the mother liquors a small quantity of
the isomeric α-(o-nitrophenyl)-α-ethyl-glutarimide of MP 170-172°C can be
obtained.
26.2 g of α-(p-nitrophenyl)-α-ethyl-glutarimide of MP 137-139°C dissolved in
ethyl acetate, are reduced in the presence of nickel with hydrogen in a
shaking flask at 50-70°C until the absorption of hydrogen falls off. The
catalyst is then filtered off with suction and the solution concentrated and
cooled, as a result of which colorless crystals of MP 146-149°C are obtained.
Recrystallization from methanol gives pure α-(p-aminophenyl)-α-ethylglutarimide
of MP 149-150°C (yield 97%).
Instead of ethyl acetate another solvent can be used in the above reduction,
such as methanol or ethanol.
The hydrochloride of MP 223-225°C is obtained by dissolving the base with
alcohol and the corresponding quantity of hydrochloric acid gas in the hot with
subsequent cooling of the solution. Colorless crystals are formed of MP 223-
225°C, which are easily soluble in water. | [Brand name]
Cytadren (Novartis);C-16038-ba;Crytraden;Doredin;Mamomit;Ormeten. | [Therapeutic Function]
Cytostatic | [World Health Organization (WHO)]
Aminoglutethimide, a weak anticonvulsant, was introduced in
1960 for use in the treatment of epilepsy. However, its adrenocortical suppressant
activity gave rise to serious adverse effects. The FDA decision in 1966 was taken in
respect of a preparation indicated in epilepsy. In 1980 preparations containing
aminoglutethimide were reintroduced in the USA exclusively for the treatment of
Cushing's disease. In 1986 they were also registered in Saudi Arabia for use in
Cushing's syndrome and for the treatment of breast cancer. In some other
countries these preparations are additionally approved for carcinoma of the
prostate. | [General Description]
Aminoglutethimide, 3-(4-aminophenyl)-3-ethyl-2,6-piperidinedione, is mainly usedto treat Cushing syndrome, a condition of adrenal steroidexcess, a use in which the P450scc inhibition of thiscompound is exploited rather than its aromatase inhibition.Aminoglutethimide is a weak inhibitor of aromataseand has been used successfully in the treatment of estrogen-dependent breast cancer. Because of the developmentof more selective aromatase inhibitors, the use ofaminoglutethimide for its ability to inhibit aromatase is notsupported. | [Mechanism of action]
This drug blocks the transformation of cholesterol into pregnenolone, and androgens into
estrogens in the adrenal glands, thus completely suppressing the production of all steroid
hormones. Aminoglutethimide is used for palliative treatment of prostate carcinomas and
post-menopausal breast carcinomas. Synonyms of aminoglutethimide are orimeten, cita�dren and others. | [Clinical Use]
Aminoglutethimide is suitable for use in Cushing’s
syndrome that results from adrenal carcinoma and in
congenital adrenal hyperplasia, in which it protects the
patient from excessive secretion of endogenous androgens.
The drug is not curative, and relapse occurs when
treatment is terminated. Since aminoglutethimide therapy
is frequently associated with mineralocorticoid deficiency,
mineralocorticoid supplements may be needed.
Aminoglutethimide and metyrapone are frequently
used in combination at lower doses of both drugs as an
adjunct to radiation or surgical therapy. | [Side effects]
Such a medical adrenalectomy is an efficacious
treatment for metastatic breast and prostate cancer,
since it diminishes the levels of circulating sex hormones.
Glucocorticoids are administered concomitantly
to suppress enhanced corticotrophin release. Cortisol is
preferable to dexamethasone in this situation because
aminoglutethimide markedly enhances the hepatic
microsomal metabolism of dexamethasone. Hepatic enzyme
induction may be responsible for the development
of tolerance to the side effects of aminoglutethimide,
such as ataxia, lethargy, dizziness, and rashes. | [Synthesis]
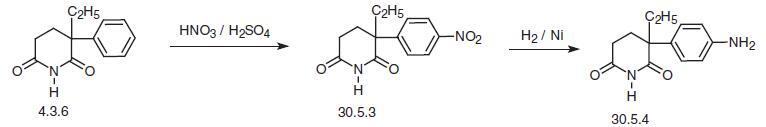
Aminoglutethimide, (±)-2-(4-aminophenyl)-2-ethylglutarimide
(30.5.4), is made by two methods, the first of which begins with glutethimide (4.3.6),
which is nitrated to form 2-(4-nitrophenyl)-2-ethylglutarimide (30.5.3). Reducing the nitro
group with hydrogen over a nickel catalyst gives the desired aminoglutethimide (30.5.4).
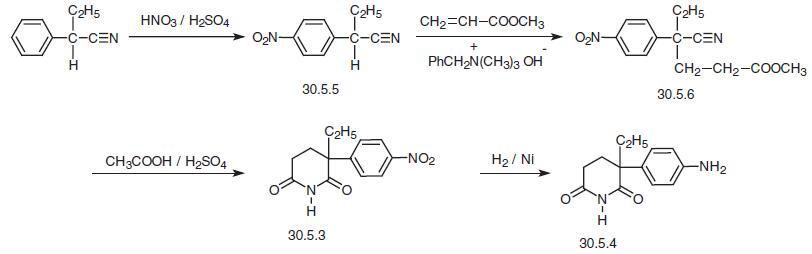
The second method starts with 2-phenylbutyronitrile, which is nitrated under analogous
conditions, forming 2-(4-nitrophenyl)butyronitrile (30.5.5). The last, in Michael addition
reaction conditions, in the presence of benzyltrimethylammonia hydroxide is added to
methylacrylate, and the obtained product undergoes acidic hydrolysis by a mixture of
acetic and sulfuric acids, during which a cyclyzation to 2-(4-nitrophenyl)-2-ethylglutarim�ide (30.5.3) occurs, and this product is reduced by hydrogen by the analogy to that
described above, to give the desired product aminoglutethimide (30.5.4) .
 | [Metabolic pathway]
Following administration of a single oral dose of 14C-
aminoglutethimide to rats, guinea pigs, rabbits, and
man, more than 89% of the dose is excreted in urine
and feces within 72h, and dogs eliminate only 51% in
this time. Extensive metabolism occurs in all species,
with N-acetylaminoglutethimide being the major
metabolite except for dogs and man. In the latter two
species, the unchanged drug is the main product
excreted. As shown in the pathways, it appears that
aminoglutethimide is metabolized by several pathways
in man and, of the ten metabolites, only two are present
in any quantity, namely N-acetylaminoglutethimide and
N-hydroxyaminoglutethimide, the latter increasing during
the course of treatment. |
|
|