| Identification | More | [Name]
1-Hydroxynaphthalene-8-sulfonic acid | [CAS]
117-22-6 | [Synonyms]
1-Hydroxynaphthalene-8-sulfonic acid
1-NAPHTHOL-8-SULFONIC ACID
1 NAPTHOL-8-SULPHONIC ACID
8-hydroxy-1-naphthalenesulfonic acid
alpha-Naphthol-8-sulfonic acid
8-hydroxynaphthalenesulphonic acid
8hydroxynaphthalene-1-sulfonic acid
1-Naphthalenesulfonic acid, 8-hydroxy-
1-NAPHTHOL-8-SULFOMIC ACID | [EINECS(EC#)]
204-180-3 | [Molecular Formula]
C10H8O4S | [MDL Number]
MFCD00065328 | [Molecular Weight]
224.23 | [MOL File]
117-22-6.mol |
| Hazard Information | Back Directory | [Chemical Properties]
1-Hydroxynaphthalene-8-sulfonic acid [117-22-6]. (8-hydroxynaphthalene-1-sulfonic acid), C10H8O4S, Mr 224.2, crystallizes as the monohydrate with mp 106℃. Sulfonation gives 1-hydroxynaphthalene-4,8-disulfonic acid, and alkali fusion with potassium hydroxide at 230℃ yields 1,8-dihydroxynaphthalene. Coupling with diazo compounds occurs in the 2- position. | [Uses]
The naphthosultone is usually satisfactory as an intermediate for further processing; however, for azo dye preparation the free naphthol must be used, for example, in C.I. Acid Black 54 and C.I. Acid Blue 158. | [Production Methods]
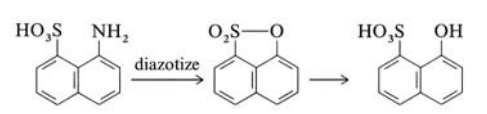
1-Aminonaphthalene-8-sulfonic acid is diazotized over 16 h at 50℃ and then heated to 80 ℃. After cooling, the precipitated naphthosultone is filtered off and washed. The sultone is heated with dilute sodium hydroxide at 100℃, which causes ring opening; the product is isolated after salting out.
 |
|
|