| Identification | More | [Name]
2,2,6,6-TETRAMETHYL-3,5-HEPTANEDIONE | [CAS]
1118-71-4 | [Synonyms]
2,2,6,6-TETRAMETHYL-3,5-HEPTANEDIONE
2,2,6,6-TETRAMETHYL-3,5-HEPTANEDIONE DIPIVALOYLMETHANE
2,2,6,6-TETRAMETHYLHEPTANE-3,5-DIONE
DIPIVALOYLMETHANE
TMHD
(CH3)3CCOCH2COC(CH3)3
2,2,6,6-Tetramethyl heptanedione
2,2,6,6-tetramethyl-5-heptanedione
Dipivaloylmethane~Dpm-H~Tmhd-H
2,2,6,6-TETRAMETHYL-3,5-HEPTANEDIONE, 95 %
2,2,6,6-Tetramethylheptane-3,5-dione,98%TMHD
3,5-Heptanedione, 2,2,6,6-tetramethyl-
dpm-h
2,2,6,6-TETRAMETHYLHEPTANE-3,5-DIONE TMHD
2,2,6,6-Tetramethyl-3,5-heptanedione, 99+%
2,2,6,6-Tetramethyl-3,5-heptanedione, 98+%
2,2,6,6-Tetramethyl-3,5-heptadione | [EINECS(EC#)]
214-268-3 | [Molecular Formula]
C11H20O2 | [MDL Number]
MFCD00008848 | [Molecular Weight]
184.28 | [MOL File]
1118-71-4.mol |
| Chemical Properties | Back Directory | [Appearance]
CLEAR COLOURLESS TO SLIGHTLY YELLOW LIQUID | [Melting point ]
>400 °C (decomp) | [Boiling point ]
72-73 °C/6 mmHg (lit.) | [density ]
0.883 g/mL at 25 °C(lit.)
| [refractive index ]
n20/D 1.459(lit.)
| [Fp ]
153 °F
| [storage temp. ]
Sealed in dry,Room Temperature | [solubility ]
Difficult to mix. | [form ]
Liquid | [pka]
11.60±0.10(Predicted) | [color ]
Clear colorless to slightly yellow | [Specific Gravity]
0.883 | [BRN ]
1447269 | [InChIKey]
YRAJNWYBUCUFBD-UHFFFAOYSA-N | [LogP]
2.818 (est) | [CAS DataBase Reference]
1118-71-4(CAS DataBase Reference) | [Storage Precautions]
Store under nitrogen | [EPA Substance Registry System]
3,5-Heptanedione, 2,2,6,6-tetramethyl- (1118-71-4) |
| Safety Data | Back Directory | [Hazard Codes ]
Xn | [Risk Statements ]
R22:Harmful if swallowed.
R40:Limited evidence of a carcinogenic effect.
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S24/25:Avoid contact with skin and eyes .
S36:Wear suitable protective clothing .
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S22:Do not breathe dust . | [RIDADR ]
UN 1993 / PGIII | [WGK Germany ]
3
| [TSCA ]
T | [HS Code ]
29141900 |
| Raw materials And Preparation Products | Back Directory | [Raw materials]
Ethanol-->Hydrochloric acid-->Ethyl acetate-->Diethyl ether-->Tetrahydrofuran-->N,N-Dimethylformamide-->Sodium hydride-->Pinacolone-->Methyl trimethylacetate-->4-Heptyn-3-one, 2,2,6,6-tetramethyl--->Benzenemethanamine, N-(1,2,2-trimethylpropylidene)--->Dipivaloylamine-->2,2-Dimethylpropanoic acid phenyl ester-->Propanamide,2,2-dimethyl-N-(phenylmethyl)--->2,3,3-TRIMETHYL-2-BUTANOL-->8-Hydroxyquinoline | [Preparation Products]
N,N-DIPHENYLQUINACRIDONE-->2,2,6,6-TETRAMETHYL-3,5-HEPTANEDIONATO POTASSIUM-->3,5-Heptanedione, 4-bromo-2,2,6,6-tetramethyl- |
| Hazard Information | Back Directory | [Chemical Properties]
CLEAR COLOURLESS TO SLIGHTLY YELLOW LIQUID | [Uses]
2,2,6,6-tetramethyl-3,5-heptanedione was used in the synthesis of α-aryl-β-diketones1 and dicyanamidobenzene-bridge diruthenium complex. | [Uses]
suzuki reaction | [Definition]
2,2,6,6-Tetramethyl-3,5-heptanedioneis a stable, anhydrous reagent. It undergoes O-additions and C-additions. In various reactions, it acts as an air-stable ligand for metal catalysts. Furthermore, it serves as a substrate for heterocycles. | [Synthesis Reference(s)]
Journal of the American Chemical Society, 100, p. 5428, 1978 DOI: 10.1021/ja00485a030 | [General Description]
Acylation, a replacement to silylation, allows the conversion of compounds that consist of active hydrogens (-OH, -SH and -NH) into esters, thioesters, and amides via the action of a carboxylic acid or derivative. The carbonyl group adjacent to the halogenated carbons is known to improve the electron capture detector (ECD) response. Acylation has several advantages:
- It enhances the stability of compounds by protecting unstable groups.
- It may confer volatility on substances like carbohydrates or amino acids, that have several polar groups that they are non-volatile and usually decompose on heating.
- It facilitates the separations not possible with underivatized compounds.
- Compounds are detectable at very low levels with an ECD.
2,2,6,6-Tetramethyl-3,5-heptanedione is a reagent used to form fragmentation-directing derivatives for GC/MS analysis. | [reaction suitability]
reagent type: derivatization reagent
reaction type: Acylations | [Synthesis]
Methyl pivalate and formamide could be used as starting materials to synthesize 2,2,6,6-Tetramethyl-3,5-heptanedione.
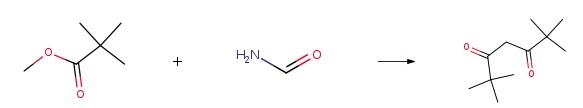 |
| Spectrum Detail | Back Directory | [Spectrum Detail]
2,2,6,6-TETRAMETHYL-3,5-HEPTANEDIONE(1118-71-4)MS
2,2,6,6-TETRAMETHYL-3,5-HEPTANEDIONE(1118-71-4)1HNMR
2,2,6,6-TETRAMETHYL-3,5-HEPTANEDIONE(1118-71-4)13CNMR
2,2,6,6-TETRAMETHYL-3,5-HEPTANEDIONE(1118-71-4)IR1
2,2,6,6-TETRAMETHYL-3,5-HEPTANEDIONE(1118-71-4)Raman
2,2,6,6-TETRAMETHYL-3,5-HEPTANEDIONE(1118-71-4)ESR
|
| Well-known Reagent Company Product Information | Back Directory | [Acros Organics]
2,2,6,6-Tetramethyl-3,5-heptanedione, 98%(1118-71-4) | [Alfa Aesar]
2,2,6,6-Tetramethyl-3,5-heptanedione, 99+%(1118-71-4) | [Sigma Aldrich]
1118-71-4(sigmaaldrich) | [TCI AMERICA]
Dipivaloylmethane,>97.0%(GC)(1118-71-4) |
|
|