| Identification | Back Directory | [Name]
Ponatinib Hydrochloride | [CAS]
1114544-31-8 | [Synonyms]
AP24534-HCl
Ponatinib-HCl
Ponatinib hydrochloride
AP24534 Mono-hydrochloride
Ponatinib Mono-hydrochloride
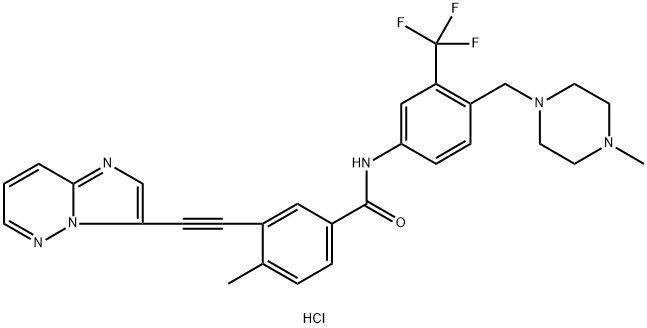
3-(imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methyl-N-(4-((4-methylpiperazin-1-yl)methyl)-3-(trifluoromethyl)phenyl)benzamide hydrochloride | [EINECS(EC#)]
1592732-453-0 | [Molecular Formula]
C29H28ClF3N6O | [MDL Number]
MFCD28656903 | [MOL File]
1114544-31-8.mol | [Molecular Weight]
569.02 |
| Chemical Properties | Back Directory | [storage temp. ]
-20°C | [solubility ]
DMF: 2 mg/ml,DMSO: 11 mg/ml,Ethanol: Slightly soluble | [form ]
A solid | [color ]
Off-white to light yellow | [InChIKey]
BWTNNZPNKQIADY-UHFFFAOYSA-N | [SMILES]
C(C1=CN=C2C=CC=NN12)#CC1C(=CC=C(C(=O)NC2C=CC(CN3CCN(C)CC3)=C(C(F)(F)F)C=2)C=1)C.Cl |
| Hazard Information | Back Directory | [Description]
In December 2012, the US FDA approved ponatinib (also referred to as AP 24534) for the treatment of adult patients with chronic phase, accelerated phase, or blast phase chronicmyeloid leukemia (CML). Ponatinib is a pan-Bcr–Abl TKI that blocks both the native (IC50=0.4 nM) and Bcr– AblT315I mutated kinases (IC50=2.0 nM) in addition to othermutated kinases in CML patients. In the Ba/F3 cell proliferation assay, ponatinib inhibits ABL and the T315I Abl mutant with IC50s of 1.2 and 8.8 nM, respectively. Ponatinib was identified by a structure-based drug design approach. Ponatinib binds to the kinase domain in a DFG-out conformation; the ethynyl moiety helps the inhibitor evade the mutant gatekeeper isoleucine residue at position 315. In addition to Abl and the T315I mutant of Abl, ponatinib inhibits VEGFR, PDGFR, FGFR, SRC, KIT, RET, TIE2, FLT3, and EPH receptors at concentrations ranging from0.1 to 20 nM. | [Originator]
Ariad (United States) | [Uses]
Ponatinib is a tyrosine kinase inhibitors (TKI) and used to treat chronic myeloid leukemia (CML) (1,2,3). Ponatinib is also used in combination with other drugs, such as forskolin, to combat TKI resistance in patient with CML (3), targeted drug in small-cell lung cancer. Potent FAK inhibitor. FGFR/VEGFR/Bcr-Abl inhibitor It is a COVID19-related research product. | [Definition]
ChEBI: Ponatinib hydrochloride is the hydrochloride salt of ponatinib. It is a potent pan inhibitor of tyrosine kinases, active in all single resistance ABL kinase mutations including the T315l mutation. It is approved for the treatment of chronic myeloid leukemia. It has a role as an antineoplastic agent and a tyrosine kinase inhibitor. It contains a ponatinib(1+). | [Brand name]
Iclusig | [Clinical Use]
Ponatinib hydrochloride (Iclusig ®), previously known as AP24534, is a multi-targeted tyrosine kinase
inhibitor approved in the US as an oral treatment for resistant or intolerant chronic myeloid leukemia
(CML) and Philadelphia chromosome-positive (Ph+) acute lymphoblastic leukemia (ALL). Ponatinib
hydrochloride was designed for treatment of tumors containing the T351I mutation which are present in
some forms of CML and resistant to traditional therapies such as imatinib. Ponatinib
hydrochloride was developed by Ariad Pharmaceauticals, and operates by a similar mechanism of action
as other tyrosine kinase inhibitors, inhibiting the enzymatic activity of BCR-ABL, an abnormal tyrosine
kinase responsible for unregulated and excess white blood cell production by bone marrow. However, the ability of ponatinib hydrochloride to target isoforms of the BCR-ABL gene typically
leading to resistance in other known tyrosine kinase inhibitors provides an alternate form of therapy not
previously available. | [Synthesis]
A significant amount of research has been devoted towards identification of a manufacturing
synthesis of ponatinib hydrochloride. A majority of methods rely on two key Sonagashira
couplings to generate the imidazo[1,2-b]pyridazin-3-ylethynyl framework. The most likely process scale method begins with 3-bromo-imidazo[1,2-b]pyridazine (135) (the Scheme). Direct Sonogashira coupling of (135) with ethynyltrimethylsilane (136) in the presence of Pd(PPh3)4 and CuI,
followed by treatment with TBAF/THF led to the desired alkynyl imidazo[1,2-b]pyridazine 138 in 71
and 94% yields, respectively. Alkyne 138 was then coupled under similar Sonogashira conditions with
functionalized aryl iodide 139 (generated in two steps from 3-iodo-4-methylbenzoic acid (140) and
commercially available piperazinyl aniline 141) providing ponatinib free base, which was then
immediately treated with EtOH/HCl at room temperature to ultimately furnish ponatinib hydrochloride
(XXI).
|
|
|