| Identification | Back Directory | [Name]
Silicic acid, ethyl ester | [CAS]
11099-06-2 | [Synonyms]
Ethyl silicate
Ethylpolysilikat
Ethyl polysilicate
Ethoxy(oxo)silanol
Ethyl Silicate 32
Ethyl Silicate 50
icic acid, ethyl ester
ETHYL SILICATE POLYMER
Ethyl Polysilicate Si-40
Silicic acid, ethyl ester
Ethyl silicate32 (Silicic acid ethyl ester)
Ethyl silicate40 (Silicic acid ethyl ester) | [EINECS(EC#)]
234-324-0 | [Molecular Formula]
C2H6O3Si | [MDL Number]
MFCD14635218 | [MOL File]
11099-06-2.mol | [Molecular Weight]
106.153 |
| Questions And Answer | Back Directory | [Physical and Chemical Properties]
Ethyl silicate (molecular formula: Si (OC2H5) 4) is a colorless liquid with special foul smell. It has the relative density being 0.933, the melting point being-77 ℃, the boiling point being 166.5 ℃, freezing point being-77 ℃, the viscosity being 0.00179Pa ? s [0.0179P (20 ℃)] and the refractive index being 1.3837 (20 ℃). It is very stable in the absence of water while it is decomposed into ethanol and silicic acid and becomes cloudy in the moist air while be clarified later and has the silicic acid being precipitated. It is soluble in alcohol, ether and other organic solvents. It is toxic with strong irritation effect on the human eye and respiratory tract. The industry applies the reaction between silicon tetrachloride and anhydrous ethanol and further distillation to obtain ethyl silicate. Ethyl silicate is mainly applied to the manufacture of heat resistant and chemical resistant coatings and preparation of organic silicone solvent. It can also be applied to organic synthesis and used as the basic raw material for making advanced crystal, optical glass processing, binders and also the insulation materials of electronics industry.
Ethyl silicate is a kind of liquid silicate and can be used as the dissolving agent of mural painting. Ethyl silicate is a clear, thin liquid which is similar to volatile solvents on the surface. After careful addition of a small amount of alcohol and water, it will be hydrolyzed into the pure silica adhesive. Before drying, it will go through viscous, adhesive intermediate stage. Applying ethyl silicate paint to the surface which is absorbent or permeable can produce a counter effect against frescoes; ethyl silicate pigment has a better performance than frescoes in term of durability and vividness of the color. Pigment must be subject to reformulation every day to be kept being fresh. Once being added with the water, you have no way to stop the hydrolysis reaction. Ethyl silicate ester has the best performance in various kinds of silicon ester. In 1931 in the UK, George ? King has for the first time introduced the ethyl silicate into the paint solvent and it was further introduced by Ralph ? Meyer (Ralph Mayer) to the United States.
| [Product Features]
Ethyl silicate is the product of the direct reaction between silicon tetrachloride and anhydrous ethanol with the reaction equation being: SiCl4 + CH3CH2OH = Si (OC2H5) 4 + 4HCl; the main component of the industrial ethyl silicate is tetraethyl orthosilicate (TEOS), and also contains a fraction of polysilicate ester and in fact, is the mixture of both compound.
Ethyl silicate itself does not have gelling properties; instead it must be in the pre-hydrolyzed in appropriate acidic or alkaline environment to form complex silicates, and then being further condensed to gel.
The binder of ethyl silicate has the following characteristics: (1) binder composition does not contain harmful impurities and low-melting substance is not generated during application; (2) it has a construction performance which can be controlled. The reaction of generation of complex silicate salt through ethyl silicate is as follows:
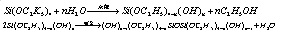
The above reaction is slow in acidic media and relatively faster in alkaline medium. In addition, ethyl silicate is not easily soluble in water and thus you should add water in the form of the aqueous solution of ethanol for causing the hydrolysis.
In the practical applications, the hydrolyzate takes a long time for generating condensed gel; therefore, we must supply some alkaline substance such as light-burning magnesium oxide powder, NH4OH for being as pro-coagulant in order to shorten the gelling time. You can alternatively apply strong organic alkaline such as aliphatic amine or heterocyclic amine as the gelling accelerator.
The heat-resistance materials with ethyl silicate as the binder has a high refractoriness and good thermal shock resistance; It has good dimensional accuracy and clean surface; it also has gas-hardening property and highly intense coagulation and hardening effect; during the process of heating the hardened material to a high temperature, the gel is gradually decomposed and evaporated and finally generates high-strength ceramic binder. However, due to that the hydrolysis reaction of ethyl silicate is difficult to control which is not suitable for being formulated and applied in the construction site; it is mainly applied to the prefabrication of the unshaped refractory and is particularly suitable for the production of multi-layer "composite" prefabricate.
Reference: Ge Lin,” brickwork manual” Beijing: Metallurgical Industry Press .1994 p. 418-419.
| [Purpose and Function]
Ethyl silicate can be used as an insulating material for electronic industry, paint, zinc coating binders, optical glass treatment agents, coagulants, organic solvents and precision casting silicone adhesive, and the model box for manufacture of metal investment casting method. The completion of the hydrolysis of ethyl silicate produces fine silica powder for the manufacture of phosphors; it can be used in organic synthesis, preparation of soluble silicon, the preparation and regeneration of catalyst; it can also be used as the cross-linking agent and intermediate also for the production of silicone.
| [Industrial production of ethyl silicate]
The main raw material for industrial preparation of ethyl silicate is silicon tetrachloride, and silicon tetrachloride is produced by the reaction of chlorine with ferrosilicon. The process is relatively simple, namely heating the ferrosilicon to above 200 ℃, then pump into chlorine gas to generate silicon tetrachloride, and then get the finished product through refined distillation. The reaction equation is as follows: Si (ferrosilicon Si) + 2Cl2-(200 ℃) → SiCl4.
The reaction equation of synthesis of ethyl silicate through the reaction between tetrachloride with ethanol and ethyl reaction is as follows: SiCl4 + 4C2H5OH → Si (OC2H5) 4 + 4HCl.
If this reaction applies anhydrous ethanol, the product is ethyl silicate; if this reaction applies water-containing industrial ethanol and in the presence of acidic or alkaline catalyst, the resulting product is the mixture of ethyl silicate and poly ethyl silicate. This is due to the presence of water in the ethanol which can cause the hydrolysis and polymerization of ethyl silicate, the reaction is as follows:
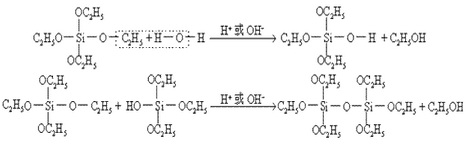
The industrial synthesis of ethyl silicate is actually the mixture of ethyl silicate and poly ethyl silicate. The polymerization degree (n) of poly-ethyl silicate is not high and in general n = 3-5, whose structural formula is as follows:
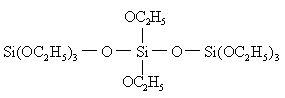
The larger the value of n, the more complex molecular form and the higher the SiO2 content is as well as the greater its cohesion has and less stability it has. The production process of industrial production of ethyl silicate is shown in the above figure.
Ethyl silicate can also be made from the esterification reaction of SiCl4 with C2H5OH at room temperature and room pressure, and then subjected to distillation according to the difference of the boiling points of components in the reaction product in order to remove the hydrogen chloride gas in the feed solution and distill the excess amount of ethanol. Finally after cooling, discoloration and filtration, we can obtain the ethyl silicate products.
The above information is edited by the Chemicalbook of Dai Xiongfeng.
| [Dangerous situations]
1. This product is of low toxicity with strong irritation effect on the eye and respiratory tract with inhalation of high concentrations having a stimulating effect which can cause damage on the lungs, liver and kidneys. Anemia has been observed in animal experiments. Symptoms caused by human’s exposure to it include: irritation to the eyes and respiratory tract; high concentrations can cause anesthetic. The anesthesia in long-term exposure may eventually lead to death.
2. It is flammable with moderate risk of combustion. The allowable concentration in air (US) is 10ppm (85mg/m3).
| [First aid]
When chemicals enter into the eyes, immediately wash with water; upon contact with skin, wash immediately with soap and water; upon a large amount of inhalation, immediately move the people away from the scene; conduct artificial respiration when necessary; if being swallowed by mistake, the people should be subject to vomiting, gastric lavage, as well as medical attention, and treatment according to the exact symptom; for severe cases, don’t conduct vomiting and go to hospital for further treatment.
| [Protective measures]
Upon operation, the people must wear protective clothing and wear protective glasses. When the skin gets wet or contamination, the worker should quickly rinse with water. If the clothes get wet or contaminated, immediately take it off to avoid the risk of combustion.
| [Medical care]
Before employment and during the periodic medical examinations, people should pay attention to check the skin, eyes, respiratory tract and liver and kidney function.
| [Measurement method]
For measurement in the air: apply resin absorption, CS2 processing and analysis with gas chromatography.
| [Storage]
Store it using glass bottles or metal tank. Avoid moisture and should be sealed for storage. Store it in the warehouse for dangerous goods.
| [Transportation requirements]
Organic narcotics; MDG Code: 84064.
| [Recommended methods of waste treatment]
Mix it with other flammable solvent for incineration together.
| [Uses]
It can be used as the adhesive of paint and pigment; also used as the cross-linking agent of silicone rubber; the binder of ceramic materials and accurate casting.
|
|
|