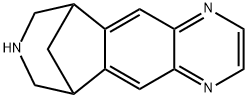
Varenicline synthesis
- Product Name:Varenicline
- CAS Number:249296-44-4
- Molecular formula:C13H13N3
- Molecular Weight:211.26

![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

249296-44-4
177 suppliers
inquiry
Yield: 95.06%
Reaction Conditions:
with ammonia in water; pH=9 - 10 at 20 - 30;Product distribution / selectivity;
Steps:
16
In a 250 ml 4 neck round bottom flask equipped with mechanical stirrer, Thermo pocket were charged DM water (30 ml) and 5,8,14-Triazatetracyclo [10.3.1.02,11.04,9]hexadeca-2(ll),3,5,7,9-pentaene Tosylate salt (10 g, having atotal purity of 99.42% obtained in example 4). The above suspension was stirred for 30 minutes at 25-30°C. To this was added 25 % aqueous ammonia solution (5 ml) to adjust the pH to 9.0-10 at 25-30°C, and MDC (30 ml) was added to this solution. The resulting mixture was stirred for 30 minutes at 20-250C. The organic layer was separated, and the aqueous layer was extracted with MDC (4x30 ml). The combined organic layer was washed with DM water (30 ml). The organic layer was treated with activated carbon (1.0 g), and stirred for 30 minutes at 25-300C. The resulting solution filtered through celite. Concentration afforded a solid product, i.e. 5,8,14- Triazatetracyclo[ 10.3.1.02, 11.04,9]hexadeca-2(ll),3,5,7,9-pentaene. (Yield-5 g (95.06 % (relative to VRN-Tosylate)), HPLC purity- 99.43 %).
References:
TEVA PHARMACEUTICAL INDUSTRIES LTD.;TEVA PHARMACEUTICALS USA, INC. WO2009/143347, 2009, A2 Location in patent:Page/Page column 25
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

249296-44-4
177 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

249296-44-4
177 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

249296-44-4
177 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

249296-44-4
177 suppliers
inquiry