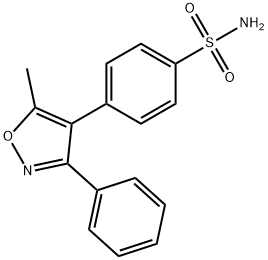
Valdecoxib synthesis
- Product Name:Valdecoxib
- CAS Number:181695-72-7
- Molecular formula:C16H14N2O3S
- Molecular Weight:314.36
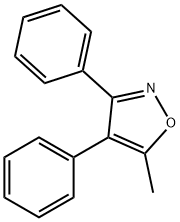
37928-17-9
307 suppliers
$7.00/1g

181695-72-7
351 suppliers
$5.00/100mg
Yield:181695-72-7 94%
Reaction Conditions:
Stage #1:5-methyl-3,4-diphenyl isoxazole with chlorosulfonic acid at 25 - 60; for 1.5 h;Large scale;
Stage #2: with ammonium hydroxide in dichloromethane at 25 - 35; for 2 h;Large scale;
Steps:
S1 synthetic intermediate I:
Feeding and reaction:Add 29.35kg of chlorosulfonic acid to a 100L explosion-proof multifunctional double-layer glass reactor,Maintain the system temperature at 25,Slowly add 7.50kg 5-methyl-3,4-diphenylisoxazole for sulfonation reaction,Maintain the temperature at 28 during the feeding process (record the temperature every 20min),After the addition is complete, maintain the temperature at 30°C and stir for 30 minutes;
The system is heated to 60°C for 1h,TLC monitors to the end of the reaction (developing agent: ethyl acetate: petroleum ether = 1:10, UV);
Extraction and washing: cooling the system to 25, adding 25kg of dichloromethane; adding 75kg of purified water and 87.5kg of dichloromethane to a 500L glass-lined reactor, cooling to below 20, adding 100L of explosion-proof multifunctional double glass dropwise The temperature of the reaction solution in the reactor is not higher than 20°C during the dropping process; after dropping, the dichloromethane and the water phase are obtained by separating the liquids. Wash twice, 50kg each time, to obtain the methylene chloride phase;
Amination reaction:The methylene chloride phase is controlled at 25°C,Add 33.75kg ammonia water dropwise,Add dropwise to control the internal temperature at 25°C (record the temperature every 10 minutes),Keep the temperature and stir for 30 minutes, and heat the reaction system to 35°C.Reaction for 1.5h, TLC monitoring,To the end of the reaction (for 2 hours, if the reaction is not complete, then conduct deviation investigation.Developing agent: ethyl acetate: petroleum ether=1:1, UV);
Post-treatment: add 112.5kg ethyl acetate to the reaction system, stir and separate the layers, extract the aqueous phase with 26.5kg dichloromethane once, combine the organic phases; wash the organic phase with 35kg purified water each time, and wash twice to obtain the organic phase;
Concentration: Transfer the organic phase to a 50L explosion-proof rotary evaporator for concentration; set the temperature to 60°C and concentrate to a vacuum of -0.08MPa. When a large amount of solids precipitate and almost no droplets drop out, stop the distillation.
Drying: load the solid into the tray, transfer it to a vacuum drying oven for drying, and dry it at a temperature of 65°C and a vacuum of -0.08MPa. After drying for 12 hours, weigh once every hour after 10 hours until the material is weighed twice. The weight difference is only 15g and the drying is stopped to obtain Parecoxib Sodium Intermediate I; the detection items and methods of Intermediate I are carried out. The spectrum of Intermediate I is shown in Figure 1, and the yield is 94%. Table 1 shows:
References:
Sichuan Pharmaceutical Preparation Co., Ltd.;Yang Ming;Li Jiacheng;Wang Jing CN111978266, 2020, A Location in patent:Paragraph 0052-0059; 0087
181696-73-1
109 suppliers
$120.50/50 mg

181695-72-7
351 suppliers
$5.00/100mg
181696-35-5
102 suppliers
$126.00/100mg

181695-72-7
351 suppliers
$5.00/100mg
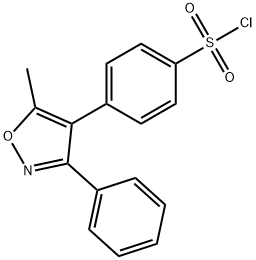
509074-26-4
115 suppliers
$15.00/250mg

181695-72-7
351 suppliers
$5.00/100mg
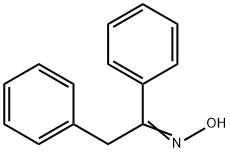
952-06-7
110 suppliers
$67.00/500mg
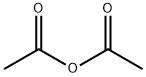
108-24-7
5 suppliers
$14.00/250ML

181695-72-7
351 suppliers
$5.00/100mg