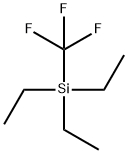
TRIETHYL(TRIFLUOROMETHYL)SILANE synthesis
- Product Name:TRIETHYL(TRIFLUOROMETHYL)SILANE
- CAS Number:120120-26-5
- Molecular formula:C7H15F3Si
- Molecular Weight:184.27
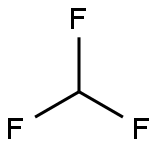
75-46-7
2 suppliers
$150.00/50 g
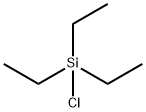
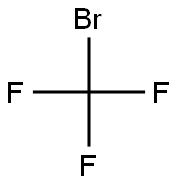
994-30-9
426 suppliers
$12.00/5g

120120-26-5
101 suppliers
$14.00/250mg
Yield:120120-26-5 71%
Reaction Conditions:
Stage #1:trifluoromethan;triethylsilyl chloride in diethyl ether at -78; for 0.166667 h;Inert atmosphere;
Stage #2: with potassium hexamethylsilazane in diethyl ether at -78 - 20; for 3 h;Inert atmosphere;
Steps:
2 Preparation of Trifluoromethyl(triethyl)silane
General procedure: Example 2
Preparation of Trifluoromethyl(triethyl)silane
To a 250 ml round bottom flask with magnetic stirrer, under argon was added 140 ml of diethyl ether.
This reaction flask was cooled to -78° C. using dry ice/acetone bath and was stirred at that temperature for ten minutes.
After ten minutes, CF3H was bubbled into this ether solution for 16 min 17 sec (2.5 g was added total at the rate of 52.5 ml/min to obtain 0.25 M solution in ether).
After 10 minutes, chlorotriethylsilane (5.32 g) was added to the reaction mixture dropwise and the resulting reaction mixture was stirred at -78° C. for ten minutes.
After ten minutes, a solution of potassium hexamethyldisilazide in ether (7.4 g in 52 ml, 0.625 M solution in ether) was added dropwise to the reaction mixture and the resulting yellowish reaction mixture was stirred at -78° C. for 2 hours and then was gradually warmed to room temperature and stirred at room temperature for 1 hour.
Ether was removed under vacuo and the residue obtained was dissolved in pentane (150 ml).
Organic layer (pentane) layer was washed with water once (1*30 ml) and was further washed with cold concentrated sulfuric acid (98%) (4*15 ml) to remove most of the siloxane and silanols formed in the reaction.
Organic layer was then washed with water (5*50 ml) until pH of the water showed neutral and dried over sodium sulfate.
Solvent (pentane) was removed under vacuo to obtain crude product which was distilled under vacuo to obtain pure (trifluoromethyl)triethylsilane 4.6 g, 71% yield. 1H NMR (400 MHz): δ 0.79 (q, 3JH-H=7.9 Hz, 6H), 1.04 (t, 3JH-H=7.9 Hz, 9H), 13C NMR (100.5 MHz): δ 0.86 (s, CH2), 6.53 (s, CH3), 132.24 (q, 1JC-F=324 Hz, CF3), 19F NMR (376.3 MHz): δ-61.3 (s), 29Si NMR (79.5 MHz): δ 7.7 (q, 2JSi-F=32 Hz, 1 Si).
References:
UNIVERSITY OF SOUTHERN CALIFORNIA;Prakash, G.K. Surya;Jog, Parag V.;Batamack, Patrice T.D.;Olah, George A. US2014/66640, 2014, A1 Location in patent:Paragraph 0058

994-30-9
426 suppliers
$12.00/5g
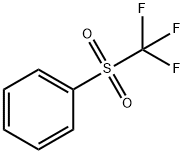
426-58-4
103 suppliers
$11.00/250mg

120120-26-5
101 suppliers
$14.00/250mg

994-30-9
426 suppliers
$12.00/5g
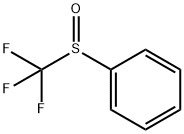
703-18-4
56 suppliers
$45.00/50mg

120120-26-5
101 suppliers
$14.00/250mg