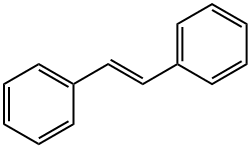
TRANS-STILBENE synthesis
- Product Name:TRANS-STILBENE
- CAS Number:103-30-0
- Molecular formula:C14H12
- Molecular Weight:180.25
Yield:103-30-0 82%
Reaction Conditions:
with diphenylsilane;N-ethyl-N,N-diisopropylamine;9-phenyl-9-phosphabicyclo[4.2.1]nonane oxide;sodium t-butyl carbonate in toluene at 110; for 24 h;Inert atmosphere;Sealed tube;Schlenk technique;Wittig Olefination;Solvent;Reagent/catalyst;Temperature;
Steps:
General procedure X: preparation of compounds All and Al6 via catalytic Wittig reaction using A2 with portion-wise addition.
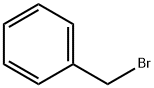
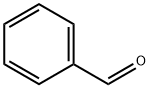
General procedure B: preparation of compounds A4-AlO and A13-A15 via catalytic Wittig reaction using DIPEA.In air, a 4 mL pressure vessel equipped with a stir-bar was charged with phosphine oxide (0.10 mmol, 10 mol %). Any other solid reagents were also added at this point, inthe following quantities: aldehyde (1 .2 mmol, 1 .2 equiv.) and organohalide (1 .0 mmol,1 .0 equiv.). The vessel was then sealed with a septum and purged with argon. Toluene(0.33 mL) and liquid reagents were introduced in the following quantities: aldehyde (1.2mmol, 1.2 equiv.), organohalide (1.0 mmol, 1.0 equiv.), DIPEA (1.2 mmol, 1.2 equiv.).Diphenylsilane (1.2 mmol, 1.2 equiv.) was introduced and the septum was replacedwith a PTFE-lined screw cap under an inert atmosphere, and the reaction was heatedat 140 00 for 24 h. The crude reaction mixture was concentrated in vacuo, and purified via flash column chromatography. When A4 was prepared in accordance with general procedure A from the reaction of benzaldehyde (122 pL, 1.2 mmol, 1.2 equiv.), benzyl bromide (120 pL, 1.0 mmol, 1.0 equiv.), diphenylsilane (223 pL, 1.2 mmol, 1.2 equiv.) and A2 (280 mg, 2.0 mmol, 2.0 equiv.) using A3a (23 mg, 0.1 mmol, 10 mol %) in toluene (1.0 mL) at 110 00 for 24 h,yield was 82% (148 mg, EIZ 95:5).
References:
DUBLIN CITY UNIVERSITY;O'BRIEN, Christopher WO2014/140353, 2014, A1 Location in patent:Page/Page column 58; 59
618-31-5
53 suppliers
$10.00/1g

103-30-0
185 suppliers
$9.00/5g
451-40-1
383 suppliers
$13.43/1gm:

103-30-0
185 suppliers
$9.00/5g