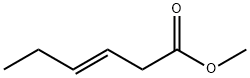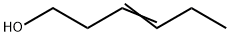
TRANS-3-HEXEN-1-OL synthesis
- Product Name:TRANS-3-HEXEN-1-OL
- CAS Number:544-12-7
- Molecular formula:C6H12O
- Molecular Weight:100.16
Yield:111-27-3 35 %Spectr. ,544-12-7 65 %Spectr.
Reaction Conditions:
with methanol;tris((2-(diphenylphosphino)ethyl)amino)ruthenium monocarbonyl;hydrogen in toluene; under 45004.5 Torr; for 18 h;Glovebox;Inert atmosphere;Autoclave;Heating;Sealed tube;
Steps:
II.II.12 11.12 Hydrogenation of unsaturated esters (methyl trans-3-hexenoate) catalyzed by Ru(L)CO with methanol as activator
In a dry argon filled glove box, a ca. 80 mL Premex stainless steel autocalve fitted with a PTFE inner chamber and a PTFE coated magnetic stirring bar was charged with the complex Ru(L)CO (0.006 mmol), methyl trans-3-hexenoate (0.6 mmol) and toluene (6 mL). Methanol was added (0.05 mL) and the autoclave was sealed. The argon atmosphere in the autoclave was re- placed with H2 by twice pressurization to 30 bar, and pressure release at room temperature. The autoclave was then pressurized with H2 gas (60 bar). The solution was heated at 130 °C (heating mantel temperature) with stirring for 18 hrs. After cooling to 0 °C, the system was vented carefully and purged for 1 minute with argon. The conversion of starting material was an- alyzed by GC on an Agilent Technologies 6890N gas chromatography system equipped with a FID detector and an Agilent Technologies DB-1 capillary column (30 m x 0.250 mm / 1 .0 pm). Another sample (0.1 mL) was diluted with CDC (0.6 mL) in an NMR tube and analyzed by 1H- NMR. Full conversion of the starting material was measured, to a mixture of ca. 65 % hex-3-en- 1 -ol, and ca. 35 % 1 -hexnaol (as well as methanol).
References:
WO2019/138000,2019,A1 Location in patent:Page/Page column 33
5747-07-9
0 suppliers
inquiry
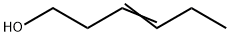
544-12-7
18 suppliers
inquiry

109-67-1
172 suppliers
$35.00/25g

107-18-6
2 suppliers
$24.60/100ml
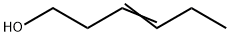
544-12-7
18 suppliers
inquiry
123-38-6
440 suppliers
$14.00/5mL

1002-28-4
131 suppliers
$10.00/250mg
928-96-1
473 suppliers
$14.00/1g
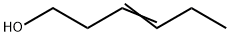
544-12-7
18 suppliers
inquiry

111-27-3
509 suppliers
$5.00/25g