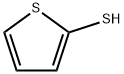
2-Thiophenethiol synthesis
- Product Name:2-Thiophenethiol
- CAS Number:7774-74-5
- Molecular formula:C4H4S2
- Molecular Weight:116.2
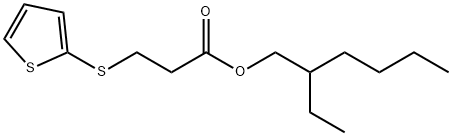
1227941-25-4
0 suppliers
inquiry

7774-74-5
312 suppliers
$6.00/1g
Yield:7774-74-5 87.3%
Reaction Conditions:
Stage #1:3-(thiophen-2-ylsulfanyl)propionic acid 2-ethylhexyl ester with sodium methylate in methanol at 20; for 1 h;Reflux;
Stage #2: with hydrogenchloride in water; pH=1 - 2 at 20;
Steps:
1.II; 2.A
Step-II: Preparation of 2-mercaptothiophene (formula 2)The product obtained from the above Step-1 (90 grams, 0.2995 mmol) was dissolved in methanol (540 mL). Sodium methoxide solution (25 %) (270 mL) was added to the reaction mass at ambient temperature. The reaction mixture was heated to reflux and maintained for 1 hour. After completion of reaction, the solvent was distilled out completely and cooled to ambient temperature. Water (450 mL) was added to the residue and washed with MDC. The product containing water layer was acidified to pH: 1-2 using concentrated hydrochloric acid. The product was extracted in to MDC (3 x 270 mL). The organic extracts was combined and washed with water. The organic layer was concentrated to obtain the product of the formula 2 as yellow oil.Output: 30.4 grams;Purity: 92.0 %; Yield: 87.3 %;1H-NMR(CDCI3): δ 3.5 l(s, I H), 6.92(q, I H), 7.08 (m, IH), 7.26(dt, IH); "C-NMR(CDCI3): δ 124.54. 127.59, 128.98, 134.01 ;MS: (m/z) = 1 14.8 (M- I ).
References:
SUVEN LIFE SCIENCES LIMITED;CHINNAPILLAI, Rajendiran;PERIYANDI, Nagarajan;JASTI, Venkateswarlu WO2010/61398, 2010, A1 Location in patent:Page/Page column 18-19; 24-25
188290-36-0
1 suppliers
inquiry

7774-74-5
312 suppliers
$6.00/1g
16629-19-9
218 suppliers
$5.00/250mg

7774-74-5
312 suppliers
$6.00/1g
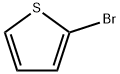
1003-09-4
479 suppliers
$10.00/25g

7774-74-5
312 suppliers
$6.00/1g
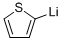
2786-07-4
16 suppliers
$50.00/25ml

7774-74-5
312 suppliers
$6.00/1g