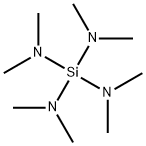
TETRAKIS(DIMETHYLAMINO)SILANE synthesis
- Product Name:TETRAKIS(DIMETHYLAMINO)SILANE
- CAS Number:1624-01-7
- Molecular formula:C8H24N4Si
- Molecular Weight:204.39
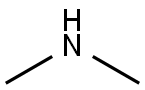
124-40-3
518 suppliers
$18.00/100ml

1624-01-7
72 suppliers
$23.80/1g
Yield: 83%
Reaction Conditions:
with tetrachlorosilane;magnesium in 1,2-dimethoxyethane;n-heptane at 80; for 4 h;
Steps:
4
Into a 300 mL four-neck flask, 6.80 g (0.28 mol) of magnesium, 60 g of n-heptane and 30 g of DME were charged. An oil bath was heated to 115° C., while stirring the resulting mixture, into a reflux state for 1 hour, and moisture in a solvent and an apparatus was allowed to react with magnesium to dehydrate the solvent and the apparatus, and then the resulting content was cooled with ice water. Into a 50 mL feed tank, 34.0 g (23 mL, 0.20 mol) of tetrachlorosilane (4CS) was charged, and when an internal temperature was decreased to 5° C., 3 mL of 4CS was charged into the 300 mL four-neck flask. In a state in which the temperature was maintained at 10° C. or lower, DMA was fed from a gas phase part of the flask thereinto at a rate of 16 mL per minute for 1 hour.First Reaction Step.The oil bath was set to 90° C., and heated until a temperature of a reaction liquid reached 80° C. or higher. On the way of the heating, a reaction between DMA hydrochloride and magnesium occurred, but no noticeable rapid increase in temperature was observed. When the internal temperature was more than 80° C., 4CS was added dropwise into the liquid at a rate of 11.5 mL per hour, and simultaneously feed of DMA to a gas phase was started at a rate of 85 mL per minute. A time of simultaneous feed of DMA was 2 hours, and a GC analysis of the reaction liquid at completion of the simultaneous feed resulted in 1.5% of 4CS, 15.8% of DME, 57.1% of n-heptane, 6.3% of (dimethylamino)trichlorosilane, 14.5% of bis(dimethylamino)dichlorosilane and 4.8% of tris(dimethylamino)chlorosilane.Second Reaction Step Further, DMA was fed for 2 hours. Then, 50.9 g (1.13 mol) of DMA in total was fed. When reduction of the temperature of the reaction liquid and loss of tris (dimethylamino) chlorosilane of an intermediate product by the GC analysis were confirmed, feed of DMA was stopped. After cooling, 181 g of the reaction liquid was obtained.Posttreatment Step The reaction liquid was filtered by a pressure filter, and a residue was washed with 30 g of n-heptane to obtain 135 g of a filtrate containing tetrakis(dimethylamino)silane (4DMAS). When the GC analysis was conducted, 33.9 g (0.17 mol) of 4DMAS was contained therein and a reaction yield was 83%. Further, when hydrolyzable chlorine was measured, a content was 140 ppm.
References:
JNC Corporation;TANAKA, Toru US2020/123180, 2020, A1 Location in patent:Paragraph 0066-0070
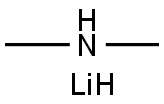
3585-33-9
53 suppliers
$54.79/100ml
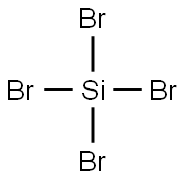
7789-66-4
89 suppliers
$57.00/5g

1624-01-7
72 suppliers
$23.80/1g
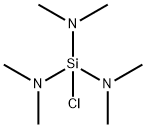
13307-05-6
41 suppliers
$305.00/10g

124-40-3
518 suppliers
$18.00/100ml

1624-01-7
72 suppliers
$23.80/1g

13307-05-6
41 suppliers
$305.00/10g
3585-33-9
53 suppliers
$54.79/100ml

1624-01-7
72 suppliers
$23.80/1g
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

124-40-3
518 suppliers
$18.00/100ml

1624-01-7
72 suppliers
$23.80/1g