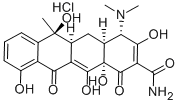
Tetracycline hydrochloride synthesis
- Product Name:Tetracycline hydrochloride
- CAS Number:64-75-5
- Molecular formula:C22H25ClN2O8
- Molecular Weight:480.9
60-54-8
304 suppliers
$14.00/1g

64-75-5
490 suppliers
$22.39/5G
Yield:-
Reaction Conditions:
with hydrogenchloride in methanol;water;
Steps:
1
Pd black (14.1 mg, 0.133 mmol, 1.75 equiv) was added in one portion to a solution of the peroxide MGC18 (48.2 mg, 0.0758 mmol, 1.0 equiv) in dioxane (3 mL) at 23 °C. An atmosphere of hydrogen was introduced by briefly evacuating the flask, then flushing with pure hydrogen (1 atm). The Pd catalyst was initially present as a fine dispersion, but aggregated into clumps within 5 min. The yellow heterogeneous mixture was stirred at 23 °C for 2 h, then was filtered through a plug of cotton. The filtrate was concentrated, affording a yellow solid. The product was purified by preparatory HPLC on a Phenomenex Polymerx DVB column (10 μM, 250 x 10 mm, flow rate 4.0 mL/min, Solvent A: methanol-0.005 N aq. HCI (1: 4), Solvent B: acetonitrile) using an injection volume of solvent A (500 μL) containing oxalic acid (10 mg) and an isochratic elution of 5% B for 2 min, then a gradient elution of 5-50% B for 20 min. The peak eluting at 11-16 min was collected and concentrated, affording (- ) -tetracycline hydrochloride as a yellow powder (16.0 mg, 44% from triketone MGC17), which was identical with natural (-)-tetracycline hydrochloride in all respects. [00166] 'H NMR (600 MHz, CD30D, hydrochloride) No. 7.50 (dd, 1H, J= 8.4,7.8 Hz, ArH), 7.13 (d, 1H, J = 7.8 Hz, ArH), 6.91 (d, 1 H, J = 8.4 Hz, ArH), 4.03 (s, 1H, CHN(CH3)2), 2.96-3.04 (m, 7H, HOC(CH3)CH, N(CH3)2), 2.91 (br dd, 1H, J= 12.6, 2.4 Hz, (CH3)2NCHCH), 2.18 (ddd, 1H, J= 12.6,6.0, 2.4 Hz, CHCHH'CH), 1.90 (ddd, IH, J= 12.6, 12.6, 12.0 Hz, CHCHH'CH), 1.60 (s, 3H, CH3) ; 13C NMR (100 MHz, CD30D) 8 195.4, 174.5, 163.8,148.3, 137.8,118.7, 116.4,116.0, 107.5, 96.5, 74.7, 71.2, 70.1, 43.5, 43.0, 35.9, 27.8, 22.9; UV max (0.1 N HCI), nm 217, 269,356; [α]D -25 1' ° (c = 0.12 in 0.1 M HCI); lit. (The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals, 12th ed. Budavari, S. ; O'Neal, M. J. ; Smith, A. ; Heckelman, P. E. ; Kinneary, J. F. , Eds. ; Merck & Co. : Whitehouse Station, NJ, 1996; entry 9337. ) UV max (0.1 N HCl), nm 220, 268, 355; [α]D = -257.9° (c = 0.5 in 0.1 M HCI); HRMS (ES) m/z calcd for (C22H240sN2+H) (at) 445.1611, found 445.1608.
References:
WO2005/112945,2005,A2 Location in patent:Page/Page column 106-107
32359-20-9
3 suppliers
inquiry

64-75-5
490 suppliers
$22.39/5G
![7-Oxabicyclo[4.1.0]hept-4-ene-3-carboxylic acid, 2,3-dihydroxy-, (1S,2R,3S,6R)-](/CAS/20210305/GIF/367954-24-3.gif)
367954-24-3
0 suppliers
inquiry

64-75-5
490 suppliers
$22.39/5G
![7-Oxabicyclo[4.1.0]hept-4-ene-1-carboxylic acid, 2,3-bis[[(1,1-dimethylethyl)dimethylsilyl]oxy]-, methyl ester, (1S,2R,3R,6S)-](/CAS/20210305/GIF/367954-37-8.gif)
367954-37-8
0 suppliers
inquiry

64-75-5
490 suppliers
$22.39/5G
852821-12-6
0 suppliers
inquiry

64-75-5
490 suppliers
$22.39/5G