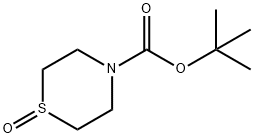
tert-butyl thiomorpholine-4-carboxylate 1-oxide synthesis
- Product Name:tert-butyl thiomorpholine-4-carboxylate 1-oxide
- CAS Number:278788-74-2
- Molecular formula:C9H17NO3S
- Molecular Weight:219.3
220655-09-4
44 suppliers
$218.00/250mg

278788-74-2
14 suppliers
inquiry
Yield:278788-74-2 99%
Reaction Conditions:
with sodium periodate in 1,4-dioxane;methanol;water at 0; for 5.5 h;
Steps:
2 tert-Butyl thiomorpholine-4-carboxylate 1-oxide
Preparation 2
tert-Butyl thiomorpholine-4-carboxylate 1-oxide
In a 250 mL round-bottom flask equipped with a magnetic stirrer were placed powdered sodium metaperiodate (5.68 g, 1.05 eq) and water (50 mL).
The mixture was stirred at RT first and then cooled to 0° C., followed by the addition of tert-butyl thiomorpholine-4-carboxylate (5.08 g, 25 mmol, 1 eq).
Then to this mixture was added dixane (30 mL) and MeOH (40 mL).
The reaction mixture was stirred at 0° C. for 5.5 hours.
It was then filtered through a Buchner funnel, the white solid was washed with CHCl3 (3λ50 mL), and the resulting water-chloroform filtrate was transferred into a separation funnel.
The lower chloroform was removed and the aqueous layer was extracted with CHCl3 (3*150 mL).
The organic phases were combined and dried over anhydrous Na2SO4 overnight.
The upper clear layer was then decanted and concentrated to give the title compound as white solid (5.41 g, 99%).
1H NMR (DMSO-d6) δ: 3.81 (d, J=13.4 Hz, 2H), 3.60 (br. s., 2H), 2.76-2.84 (m, 2H), 2.65-2.71 (m, 2H), 1.41 (s, 9H)
References:
US2015/166518,2015,A1 Location in patent:Paragraph 0070; 0071

24424-99-5
839 suppliers
$13.50/25G

278788-74-2
14 suppliers
inquiry