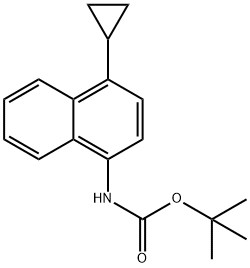
TERT-BUTYL 4-CYCLOPROPYLNAPHTHALEN-1-YLCARBAMATE synthesis
- Product Name:TERT-BUTYL 4-CYCLOPROPYLNAPHTHALEN-1-YLCARBAMATE
- CAS Number:1533519-91-3
- Molecular formula:C18H21NO2
- Molecular Weight:283.36
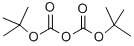
24424-99-5
865 suppliers
$13.50/25G
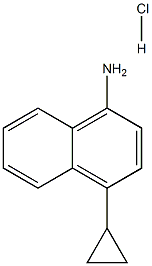
1533519-92-4
154 suppliers
$6.00/250mg

1533519-91-3
14 suppliers
$336.00/250mg
Yield:-
Reaction Conditions:
with triethylamine in ethanol;
Steps:
7 Exam le 7: Synthesis of Compound 11 - method 2
[0349] Cyclopropylmagnesium bromide was added to a solution of bromonaphthalene in tetrahydrofuran stirred at 0-5 °C in the presence of catalytic amount of [1,3- bis(diphenylphosphino)propane]dichloronickel(II) to form cyclopropylnaphthalene, which was diluted with ethyl acetate and washed. [0350] Cyclopropylnaphthalene (Compound 6) was dissolved in dichloromethane, then nitric acid was added at 0 °C and the reaction mixture was allowed to warm to ambient temperature. After reaction completion, the mixture was neutralized with sodium bicarbonate then washed with water, and l-cyclopropyl-4-nitronaphthalene (Compound 7) was used in the next step without further purification. [0351] Compound 7 was dissolved in methanol, and hydrogenated with hydrazine hydrate at reflux temperature. The crude l-amino-4-cyclopropylnaphthalene (Compound 8) was dissolved in ethanol and reacted with di-tert-butyl dicarbonate in the presence of triethylamine to give tert- butyl 4-cyclopropylnaphthalen-l-ylcarbamate (Compound 14)which was precipitated from methyl tert-butyl ketone. [0352] The protecting group in Compound 14 was removed by hydrochloric acid in ethanol to afford amino-4-cyclopropylnaphthalene which crystallized as an hydrochloride salt (compound 8-B). [0353] 4-Cyclopropylnaphthalen-l -amine hydrochloride (Compound 8-B) was dissolved in dichloromethane and treated with thiophosgene in the presence of sodium hydroxide to generate the corresponding isothiocyanate intermediate COmpound 9. [0354] l-Cyclopropyl-4-isothiocyanatonaphthalene was solvent exchanged with DMF and condensed with amino guanidine hydrochloride to generate the corresponding substituted thiosemicarbazide (Compound 13). Compound 13 was heated in the presence of aqueous sodium hydroxide to form Compound 11 , which was purified by crystallization from a mixture of ethanol and water, then recrystallized from a mixture of dimethylformamide and water. [0355] While preferred embodiments of the present invention have been shown and described herein, it will be obvious to those skilled in the art that such embodiments are provided by way of example only. Numerous variations, changes, and substitutions will now occur to those skilled in the art without departing from the invention. It should be understood that various alternatives to the embodiments of the invention described herein may be employed in practicing the invention. It is intended that the following claims define the scope of the invention and that methods and structures within the scope of these claims and their equivalents be covered thereby.
References:
WO2014/8295,2014,A1 Location in patent:Paragraph 0349-0355