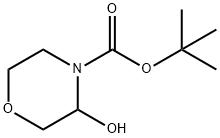
tert-butyl 3-hydroxymorpholine-4-carboxylate synthesis
- Product Name:tert-butyl 3-hydroxymorpholine-4-carboxylate
- CAS Number:1097871-14-1
- Molecular formula:C9H17NO4
- Molecular Weight:203.24
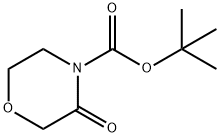
142929-48-4
64 suppliers
$56.00/250mg

1097871-14-1
15 suppliers
inquiry
Yield:1097871-14-1 80%
Reaction Conditions:
with diisobutylaluminium hydride in diethyl ether;toluene at -78 - 20;
Steps:
97.1
tert-Butyl 3-oxomorpholine-4-carboxylate (8.65 g, 43.0 mmol; prepared as described in Chandrakumar, N.S.; et al.; J Med Chem 35:2928 (1992)) was dissolved in Et2O (45 mL) and cooled to -78°C. The solution was treated dropwise with DIBAL-H (1.5M solution in toluene; 29.2 mL, 43.8 mmol), and the mixture was stirred at -78°C for 90 minutes. The mixture was then warmed to ambient temperature and stirred overnight. The reaction was quenched with saturated NH4Cl (50 mL) and stirred for 20 minutes. The reaction was diluted with ethyl acetate (100 mL) and Rochelle's salt (50 mL of 0.5N solution) and then stirred for 20 minutes. Additional Rochelle's salt (75 mL, 0.5N) was added, and then the reaction was stirred for 20 minutes and separated. The aqueous portion was washed with ethyl acetate (2 X). Then the combined organics were washed twice with Rochelle's salt (0.5 N), saturated NaHCO3, saturated NaCl, dried over Na2SO4 and concentrated to an oil, tert-butyl 3-hydroxymorpholine-4-carboxylate (7.0 g, 80%).
References:
WO2009/6567,2009,A2 Location in patent:Page/Page column 94

24424-99-5
833 suppliers
$13.50/25G

1097871-14-1
15 suppliers
inquiry