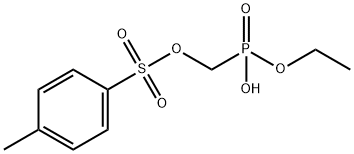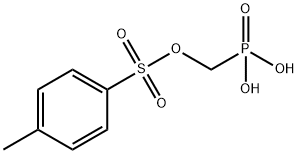
Tenofovir Impurity 38 synthesis
- Product Name:Tenofovir Impurity 38
- CAS Number:161760-09-4
- Molecular formula:C8H11O6PS
- Molecular Weight:266.21
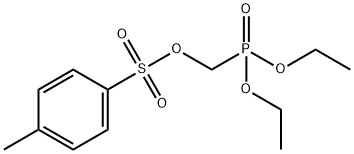
31618-90-3
429 suppliers
$5.00/5g

161760-09-4
26 suppliers
inquiry
Yield:161760-09-4 98%
Reaction Conditions:
with trimethylsilyl bromide in acetonitrile at 30; for 24 h;Cooling with ice;Solvent;Reagent/catalyst;Temperature;
Steps:
1 Preparation of the compound p-toluenesulfonyloxymethylphosphonic acid as shown in formula (III):
15 g (0.046 mol) of diethyl p-toluenesulfonyloxymethylphosphonate was added to a 1 L reaction flask.450ml acetonitrile, stirred,56.4 g (0.37 mol) of trimethylbromosilane was slowly added dropwise under ice bath, and the addition was completed.The temperature was raised to 30 ° C, and the reaction was carried out at 30 ° C for 24 hours. The reaction was completed. The acetonitrile was concentrated under reduced pressure. 100 ml of toluene was added to the concentrate and transferred to a 500 ml three-necked flask. The temperature was lowered to 10 to 20 ° C in an ice bath, and then 100 ml of pre-mixed was added. Cold water, heat at 10 ~ 20 ° C for 10 min, let stand, layer, remove the organic layer.The aqueous layer was washed three times with 100 ml of ethyl acetate, and kept at 10 to 20 ° C. After washing, the aqueous layer was removed, and the ethyl acetate layer was combined. Then, 100 ml of saturated brine was added to the ethyl acetate layer, and the mixture was stirred at 10 to 20 ° C for 5 to 5 10 min, let stand, layer, remove the water layer.The organic layer was added to the ethyl acetate layer and dried over anhydrous magnesium sulfate. The mixture was stirred at 10 to 20 ° C for 10 min, filtered, and ethyl acetate was evaporated in vacuo to give 12.3 g of compound (III) as a colorless transparent oily liquid, yield 98%,
References:
CN108178772,2018,A Location in patent:Paragraph 0019; 0020; 0021
35717-98-7
31 suppliers
inquiry

161760-09-4
26 suppliers
inquiry