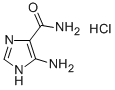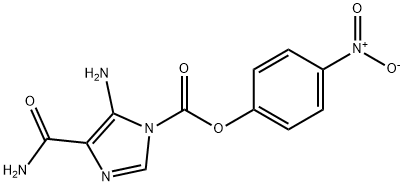
Temozolomide Impurity synthesis
- Product Name:Temozolomide Impurity
- CAS Number:196806-10-7
- Molecular formula:C11H9N5O5
- Molecular Weight:291.22
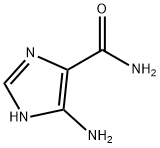
360-97-4
380 suppliers
$5.00/250mg
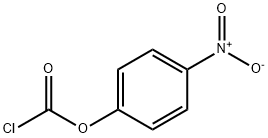
7693-46-1
421 suppliers
$18.00/5g

196806-10-7
7 suppliers
inquiry
Yield:196806-10-7 92%
Reaction Conditions:
Stage #1: 5-Aminoimidazole-4-carboxamidewith triethylamine in dichloromethane at 25; for 0.166667 h;
Stage #2: 4-Nitrophenyl chloroformate in dichloromethane at 25; for 22 h;
Steps:
A Preparation of Step A Intermediate 2
To a 5 L three-necked round bottom flask equipped with a thermometer, 5-aminoimidazole-4-carboxamide A (80 g, 634.32 mmol), dichloromethane (1920 mL), triethylamine (176.82 mL, 1268.63 mmol), After stirring at 25 ° C for 10 minutes, the temperature of the reaction system was lowered to below 0 ° C. After 10 minutes, 4-nitrophenyl chloroformate (255.71 g, 1268.36 mmol) dissolved in 1280 mL of dichloromethane was added dropwise. After reacting for 4 hours at 0 ° C or lower, the temperature was controlled at 25 ° C for 18 hours. The reaction solution was suction filtered with a Buchner funnel, and the obtained cake was washed with 1000 mL of dichloromethane and 200 ml of water for 1 hour, and filtered again. The filter cake was washed with dichloromethane and dried at room temperature to obtain a yellow solid product. . Yield: 92%, melting point: decomposition at 200 ° C.
References:
CN103626772,2016,B Location in patent:Paragraph 0006; 0009; 0027; 0028-0031