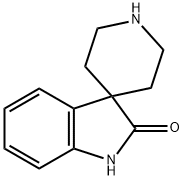
SPIRO[INDOLINE-3,4'-PIPERIDIN]-2-ONE synthesis
- Product Name:SPIRO[INDOLINE-3,4'-PIPERIDIN]-2-ONE
- CAS Number:252882-61-4
- Molecular formula:C12H14N2O
- Molecular Weight:202.25
![1'-benzylspiro[indoline-3,4'-piperidin]-2-one](/CAS/20200331/GIF/1086063-19-5.gif)
1086063-19-5
7 suppliers
inquiry
![SPIRO[INDOLINE-3,4'-PIPERIDIN]-2-ONE](/CAS/GIF/252882-61-4.gif)
252882-61-4
61 suppliers
$59.00/100mg
Yield:252882-61-4 90.2%
Reaction Conditions:
with hydrogen;palladium 10% on activated carbon in methanol at 20; for 15 h;
Steps:
1.1
Process 1Production of spiro(indole-3,4'-piperidine)-2(1H)-oneSpiro(indole-3,4'-piperidine)-2(1H)-one was produced by the method described below.Under an argon atmosphere, a tetrahydrofuran solution (20 mL) of oxindole (3.00 g, 22.5 mmol) and a tetrahydrofuran solution (20 mL) of benzylbis(2-chloroethyl)amine (5.20 g, 22.5 mmol) were added sequentially to a tetrahydrofuran solution (100 mL) of sodium hydride (1.60 g, 67.6 mmol) at room temperature. The mixture was stirred at the same temperature for 1 hour and further stirred at 90° C. for 3 hours. The reaction solution was cooled to room temperature, and then added with a tetrahydrofuran solution (10 mL) of sodium hydride (0.540 g, 22.5 mmol) and stirred further at 90° C. for 12 hours. The reaction solution was added with saturated ammonium-chloride aqueous solution and stirred at room temperature for 10 minutes. The mixed solution was poured into the mixed solution of a saturated aqueous solution of sodium hydrogen carbonate and brine, then extracted with ethyl acetate. The organic layer was dried with anhydrous sodium sulfate, followed by a vacuum concentration. The resultant residue was purified by silica-gel chromatography (chloroform:methanol=20:1) and 1'-benzylspiro(indole-3,4'-piperidine)-2(1H)-one (2.94 g, 44.6%) was obtained as a yellow amorphous solid.1H-NMR (400 MHz, CDCl3) δ; 1.78-1.93 (m, 2H), 1.95-2.06 (m, 2H), 2.65-2.77 (m, 2H), 2.87-3.00 (m, 2H), 3.69 (s, 2H), 6.90 (d, J=7.6 Hz, 1H), 7.02(t, J=7.6 Hz, 1H), 7.20 (t, J=7.6 Hz, 1H), 7.26 (t, J=6.2 Hz, 1H), 7.34 (t, J=7.6 Hz, 2H), 7.40-7.41(m, 3H), 8.72 (s, 1H).To a methanol solution (5 mL) of 1'-benzylspiro(indole-3,4'-piperidine)-2(1H)-one (300 mg, 1.03 mmol), 10% palladium carbon (30.0 mg) was added. The mixture was stirred under a hydrogen atmosphere at room temperature for 15 hours. The reaction solution was filtered using celite followed by a vacuum concentration, and spiro(indole-3,4'-piperidine)-2(1H)-one (187 mg, 90.2%) was obtained as a colorless amorphous solid.1H-NMR (400 MHz, CDCl3) δ; 1.73-1.78 (m, 2H), 1.88-1.94 (m, 2H), 3.06-3.12 (m, 2H), 3.35-3.41 (m, 2H), 6.92 (d, J=7.7 Hz, 1H), 7.04 (t, J=7.7 Hz, 1H), 7.22 (t, J=7.7 Hz, 1H), 7.42 (d, J=7.7 Hz, 1H), 8.70 (br, 1H).
References:
US2008/306102,2008,A1 Location in patent:Page/Page column 12
![1,2-DIHYDRO-2-OXO-SPIRO[3H-INDOLE-3,4'-PIPERIDINE]-1'-CARBOXYLIC ACID 1,1-DIMETHYLETHYL ESTER](/CAS/GIF/252882-60-3.gif)
252882-60-3
100 suppliers
$30.00/100mg
![SPIRO[INDOLINE-3,4'-PIPERIDIN]-2-ONE](/CAS/GIF/252882-61-4.gif)
252882-61-4
61 suppliers
$59.00/100mg
59-48-3
481 suppliers
$5.00/1g
![SPIRO[INDOLINE-3,4'-PIPERIDIN]-2-ONE](/CAS/GIF/252882-61-4.gif)
252882-61-4
61 suppliers
$59.00/100mg