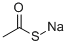
SODIUMTHIOLACETATE synthesis
- Product Name:SODIUMTHIOLACETATE
- CAS Number:34832-35-4
- Molecular formula:
- Molecular Weight:0
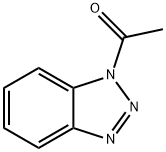
18773-93-8
38 suppliers
$18.00/1g

34832-35-4
4 suppliers
inquiry
Yield: 97%
Reaction Conditions:
with sodium hydrogen sulfide in water at 20; for 5 h;Green chemistry;
Steps:
General Procedure
General procedure: To a stirred solution of NaSH (9 mmol, 3 equiv.) in water (4 mL) was added N-acyl benzotriazole 1 (3 mmol, 1 equiv.). The reaction mixture was stirred at room temperature for 3 h. After completion of the reaction, HCl (2N) was added dropwise to acidify the solution. The precipitated solid was collected by vacuum filtration, washed with water (10 mL), and dried. In case of oily residue, the aqueous solution was decanted, and the residue was dissolved in EtOAc (15 mL). This solution was washed with 10% HCl (2 × 10 mL), brine (10 mL), dried over MgSO4, and concentrated under reduced pressure. These compounds are oils at room temperature but usually solidify after refrigeration.
References:
Elagawany, Mohamed;Hegazy, Lamees;Elgendy [Tetrahedron Letters,2019,vol. 60,# 30,p. 2018 - 2021] Location in patent:supporting information
507-09-5
315 suppliers
$12.00/5g

34832-35-4
4 suppliers
inquiry