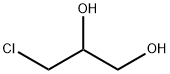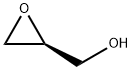
(S)-Oxiranemethanol synthesis
- Product Name:(S)-Oxiranemethanol
- CAS Number:60456-23-7
- Molecular formula:C3H6O2
- Molecular Weight:74.08

107-18-6
2 suppliers
$24.60/100ml

60456-23-7
243 suppliers
$8.00/1g
Yield:60456-23-7 72%
Reaction Conditions:
with L-(+)-diisopropyl tartrate;Cumene hydroperoxide;titanium(IV) isopropylate in dichloromethane at 0; for 48 h;Product distribution / selectivity;Molecular sieve;
Steps:
1.b Example 1
1-b The same reaction was repeated but this time it was carried out in a homogeneous medium, using 470 mg of titanium tetraisopropoxide (1.68 mmol) instead of the solid prepared by impregnation with silica with this same compound (Example 1-a), 2 g of powdered zeolite 3 , 64 ml of dichloromethane and 2.0 ml of a 1.0M solution of diisopropyl (+)-tartrate (2.0 mmol) in dichloromethane. The combined mixture is stirred in a round-bottomed flask for 4 hours at 0° C. 1.86 g of allyl alcohol (32 mmol) and then, 30 minutes later, 11.5 ml of 80% technical grade cumyl hydroperoxide (Aldrich), dried beforehand over 3 sieve (approximately 64 mmol), are subsequently introduced successively. This corresponds to a Ti/allyl alcohol molar ratio of 5/100. The combined mixture is left to stir for 48 hours at 0° C. in order for the epoxidation reaction to take place. Glycidol is then produced with yield of 72%, a selectivity of 95% and an enantiomeric excess of 80% (predominantly S-glycidol).
References:
Centre National de la Recherche Scientifique (C.N.R.S.) US6642170, 2003, B1 Location in patent:Page/Page column 9

107-18-6
2 suppliers
$24.60/100ml
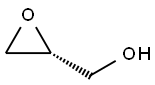
57044-25-4
251 suppliers
$6.00/1g

60456-23-7
243 suppliers
$8.00/1g
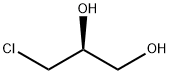
60827-45-4
270 suppliers
$14.00/5g

60456-23-7
243 suppliers
$8.00/1g
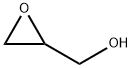
556-52-5
267 suppliers
$17.67/1gm:

56-81-5
1663 suppliers
$5.00/25g

60456-23-7
243 suppliers
$8.00/1g