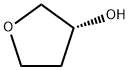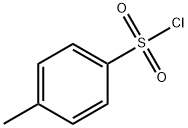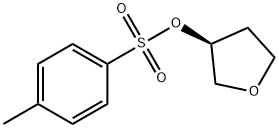
(S)-3-P-MESYLOXYTETRAHYDROFURAN synthesis
- Product Name:(S)-3-P-MESYLOXYTETRAHYDROFURAN
- CAS Number:112052-11-6
- Molecular formula:C11H14O4S
- Molecular Weight:242.29
Yield:112052-11-6 83%
Reaction Conditions:
with triethylamine in dichloromethane at 0 - 20; for 8 h;Product distribution / selectivity;
Steps:
17.5
Step 5: Preparation of (S)-tetrahydrofuran-3-yl 4-methylbenzenesulfonate To a solution of (R)-tetrahydrofuran-3-ol (3 g, 34.1 mmole) and Et3N (6.9 g, 68.2 mmole) in DCM (40 mL) at 0° C. was added 4-methylbenzene-1-sulfonyl chloride (7.2 g, 37.5 mmole. The mixture was stirred at room temperature for 8 h. The mixture was washed with water, dried over MgSO4, filtered, and concentrated. The residue was purified by flash chromatography (silica gel, 15% ethyl acetate in hexane) to give the title compound (6.8 g, 83% yield). MS (ESI) m/z: Calc. 242.1 (M+). Found: 243.1 (M++1).
References:
Cystic Fibrosis Foundation Therapeutics, Inc. US8334292, 2012, B1 Location in patent:Page/Page column 79-80
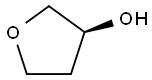
86087-23-2
666 suppliers
$5.00/1g
104-15-4
734 suppliers
$133.00/500g

112052-11-6
52 suppliers
inquiry

86087-23-2
666 suppliers
$5.00/1g

98-09-9
347 suppliers
$12.00/25 g

112052-11-6
52 suppliers
inquiry