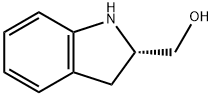
(S)-(+)-2-INDOLINEMETHANOL synthesis
- Product Name:(S)-(+)-2-INDOLINEMETHANOL
- CAS Number:27640-33-1
- Molecular formula:C9H11NO
- Molecular Weight:149.19
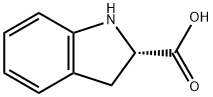
79815-20-6
417 suppliers
$17.46/5G

27640-33-1
48 suppliers
$45.00/10mg
Yield:27640-33-1 96%
Reaction Conditions:
with lithium aluminium tetrahydride in tetrahydrofuran;diethyl ether;Inert atmosphere;
Steps:
Chiral Auxiliaries and Phosphoramidites
To a solution of (2S)-dihydro-1 H-indole-2-carboxylic acid 1 (1 .63 g, 10.0 mmol) in ether (50 mL) was added a solution of LiAIH4 in THE (2M, 7.5 mL, 15.0 mmol) under argon, and the mixture was stirred overnight. After completion of the reaction, the mixture was quenched with Na2SO41 0H20. The solidwas filtered off and washed with ethyl acetate, and the filtrate was dried over anhydrous Na2SO4. Themixture was filtered, and the solvent evaporated to give a residue, which was subjected to flash silica gelcolumn purification on an 1500 (hexane/ethyl acetate, 10-70%) to give 1 .44 g (96%) of compound 2 as agray solid. 1H NMR (500 MHz, CDCI3 ppm): O7.10 (1H, d, J7.5 Hz), 7.04 (1H, t, J7.5 Hz), 6.75 (1H, t, J7.5 Hz), 6.69 (1 H, d, J 7.5 Hz), 4.10-4.06 (2H, m), 3.75 (1 H, dd, J 11.0, 4.0 Hz), 3.60 (1 H, dd, J 11 .0, 6.0Hz), 3.12(1 H, dd, J 16.0, 9.0 Hz), 2.87(1 H, dd, J 16.0, 8.0 Hz); ESI MS for C9HllNOcalculated 149.2, observed [M÷H] 150.1.
References:
WO2019/6455,2019,A1 Location in patent:Page/Page column 37
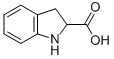
16851-56-2
101 suppliers
inquiry

27640-33-1
48 suppliers
$45.00/10mg
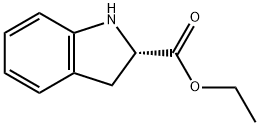
82923-81-7
30 suppliers
$200.00/500mg

27640-33-1
48 suppliers
$45.00/10mg
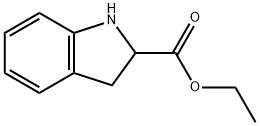
50501-07-0
57 suppliers
$60.00/100mg

27640-33-1
48 suppliers
$45.00/10mg
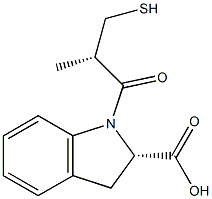
78779-29-0
0 suppliers
inquiry

27640-33-1
48 suppliers
$45.00/10mg