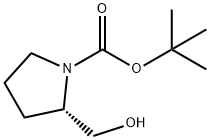
BOC-L-Prolinol synthesis
- Product Name:BOC-L-Prolinol
- CAS Number:69610-40-8
- Molecular formula:C10H19NO3
- Molecular Weight:201.26
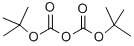
24424-99-5
858 suppliers
$13.50/25G
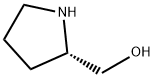
23356-96-9
461 suppliers
$11.19/5G

69610-40-8
397 suppliers
$6.00/5g
Yield:69610-40-8 100%
Reaction Conditions:
with triethylamine in ethyl acetate at 0 - 20; for 11.5 h;
Steps:
1.1; 2.1 Example 1; Preparation of 2-(R)-methyl-pyrrolidine-1-carboxylic Tert-butyl Ester (5); Step 1: Preparation of 2-(S)-hydroxymethyl-pyrrolidine-1-carboxylic Acid tert-butyl Ester (2)
Step 1: Preparation of 2-(S)-hydroxymethyl-pyrrolidine-1-carboxylic Acid tert-butyl Ester (2) A solution of (S)-prolinol (1, 50 g, 0.49 mol) in ethyl acetate (250 ML) was cooled to 0° C. triethylamine (139 ML, 101 g, 1 mol) was added dropwise to this cold reaction mixture while maintaining the reaction temperature at below 0° C. A solution of tert-butoxycarbonyl anhydride (126 ML, 119.7 g, 0.54 mol) in ethyl acetate (100 ML, EtOAc) was added dropwise to the reaction mixture while maintaining reaction temperature below 0° C. (~30 minutes).The reaction mixture was stirred at room temperature overnight (~11 hours). TLC showed absence of starting material (on Si gel, EtOAc, I2).The product was detected by HPLC at 205 nm.Reaction was quenched with 1 M aqueous H3PO4 (300 ML).The organic layer was separated and washed with 1 M aqueous H3PO4 (3*300 ML) followed by saturated aqueous NaHCO3 (3*200 ML), dried (MgSO4), filtered and concentrated to leave an oily residual product (109 g, 99.5 g for 100% yield).1H NMR (CDCl3): δ 1.47 (s, 9H, 3*CH3), 1.81 (m, 2H, CH2), 2.01 (m, 2H, CH2), 3.38 (m, 2H, CH2), 3.61 (m, 2H, CH2) and 3.97 (m, 1H, CH); [M+H]+at m/z 202.
References:
US2004/171845,2004,A1 Location in patent:Page 7
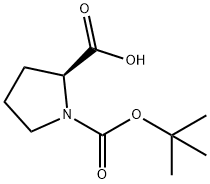
15761-39-4
612 suppliers
$5.00/10g

69610-40-8
397 suppliers
$6.00/5g
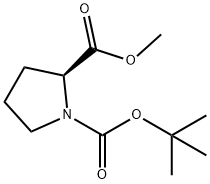
59936-29-7
229 suppliers
$6.00/5g

69610-40-8
397 suppliers
$6.00/5g
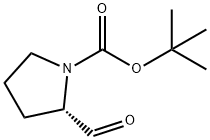
69610-41-9
267 suppliers
$9.00/1g

69610-40-8
397 suppliers
$6.00/5g

23356-96-9
461 suppliers
$11.19/5G

69610-40-8
397 suppliers
$6.00/5g