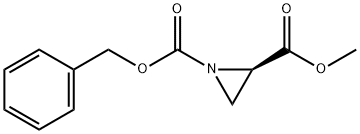
(R)-1-benzyloxycarbonyl-2-methoxycarbonylaziridine synthesis
- Product Name:(R)-1-benzyloxycarbonyl-2-methoxycarbonylaziridine
- CAS Number:154632-86-7
- Molecular formula:C12H13NO4
- Molecular Weight:235.24
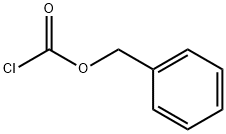
501-53-1
397 suppliers
$10.00/1g
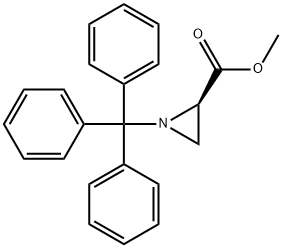
160233-42-1
77 suppliers
$11.00/250mg

154632-86-7
22 suppliers
inquiry
Yield:154632-86-7 108.5 g
Reaction Conditions:
Stage #1: 1-(trityl)aziridine-2R-carboxylic acid methyl esterwith trifluoroacetic acid in chloroform at 0; for 1 h;
Stage #2: benzyloxycarbonyl chloridewith Sodium hydrogenocarbonate in diethyl ether at 0; for 2 h;
Steps:
1.2 Step 2): Preparation of 16 (R)-1-benzyl 2-methyl aziridine-1,2-dicarboxylate
328.4 g of 17 (R)-methyl 1-tritylaziridine-2-carboxylate was dissolved in 1.4 L of 8 chloroform and the reaction solution was cooled to 0°C, to which 462 mL of 18 trifluoroacetic acid was then slowly added. The reaction mixture was stirred for 1 hour, to which 2 L of 11 water was then added, followed by stirring for 10 min and removal of the organic layer. The aqueous layer was neutralized with sodium hydrogen carbonate and used in subsequent reactions without further purification. 2 L of 19 diethyl ether and 120.5 g of 20 sodium hydrogen carbonate were added to the aqueous layer, and the reaction solution was cooled to 0°C, to which 165 mL of 21 benzyl chloroformate was then slowly added dropwise. The reaction mixture was stirred for another 2 hours and the aqueous layer was removed. The organic layer was dried over magnesium sulfate, concentrated and dried under reduced pressure, and purified by column chromatography, thereby affording 108.5 g of the title compound. 1H NMR (400 MHz, DMSO) : 7.32-7.36(m, 5H), 5.13(s, 2H), 3.09(dd, J=3.2, 5.4Hz, 1H), 2.58(dd, J=1.2, 3.2Hz, 1H), 2.47(dd, J=1.2, 5.4Hz, 1H),
References:
EP2351567,2017,B1 Location in patent:Paragraph 0071-0072
103539-32-8
31 suppliers
$454.50/1g

501-53-1
397 suppliers
$10.00/1g

154632-86-7
22 suppliers
inquiry